The ocean “acidification” narrative that claims humans are gradually lowering pH levels in sea water with their CO2 emissions may rest on presumptions, hypotheticals, and confirmation bias — not robust, observational scientific evidence.
A paper by Wei et al. (2015) published a year ago in the Journal of Geophysical Research effectively illustrates the vacuousness of the ocean “acidification” paradigm.
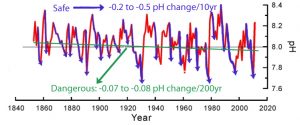
In the paper, the authors assert that “model calculations” (yes, calculations from modeling) have indicated oceanic pH levels may have decreased (i.e., lowered pH = less alkaline = more “acidic”) since the 1800s by a total of about 0.1 as consequence of the rise in anthropogenic CO2 emissions. This overall pH-lowering “trend” of less than 0.1 since the industrial era began is “predicted” to “potentially threaten the existence and development of many marine calcareous organisms”. Again, it’s the 150-year -0.1 trend in pH-lowering — which the authors admit is subject to “large errors” in measurement — that threatens the oceanic biosphere according to modeled predictions. In contrast, large natural pH drops of -0.2 to -0.5 occurring on 10-year timescales do not threaten “marine calcareous organisms.” Here are the key points from the paper:
Wei et al., 2015 Ocean acidification is predicted to reduce the saturation state of carbonate minerals in seawater and potentially threaten the existence and development of many marine calcareous organisms, such as calcareous microorganisms and corals. Model calculations have indicated an overall decrease in global seawater pH of 0.1 relative to the preIndustrial era value, and a further pH reduction of 0.2–0.3 over the next century.
We here estimate the OA rates from the two long (>150 years) annually resolved pH records from the northern SCS (this study) and the northern GBR [Great Barrier Reef], and the results indicate annual rates of -0.00039 +/- 0.00025 yr and -0.00034 +/- 0.00022 yr for the northern SCS [South China Sea] and the northern GBR [Great Barrier Reef], respectively. … [T]hese two time-series do not show significant decreasing trend for pH. Despite such large errors, estimated from these rates, the seawater pH has decreased by about 0.07–0.08 U over the past 200 years in these regions. … The average calculated seawater pH over the past 159 years was 8.04 [with a] a seawater pH variation range of 7.66–8.40.
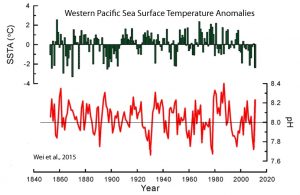
Below is the “money” graph from the paper that depicts sea surface temperature anomalies (top) and decadal-scale variations ranging between 7.66 and 8.40 in seawater pH (bottom) since the 1850s for the West Pacific Ocean:
First, notice that the Wei et al. (2015) sea surface temperature (SST) graph (top, green font) indicates there has been a rather significant cooling trend in the Western Pacific since the 1980s, and that SSTs are no warmer today than they were in the 1850s. This is consistent with other reconstructions that show modern SSTs in the region (NW Pacific) may still be a full degree C colder than they were during the Medieval Warm Period, and multiple degrees colder than they were a few thousand years ago (Yamamoto et al., 2016, Rosenthal et al., 2017).
But the bottom graph (red font) of pH variability since the mid-19th century is even more cogent. Notice that pH levels fluctuate between about 7.7 and 8.4 throughout the 150+ years, with many of the amplitudes found in the rises and falls in pH occurring at rates of + or – 0.2 to 0.5 per decade. So we are apparently expected to believe that changes in pH of + or – 0.2 to 0.5 per decade are not dangerous or “predicted” to “potentially threaten the existence and development of many marine calcareous organisms”, but the overall “acidic” or pH-lowering “trend” of less than -0.1 over 150 years is supposed to be dangerous to the oceanic biosphere. Below is an annotated version of this same graph brandishing this flagrant contradiction.
Daniel Cressey, who has previously helped expose a growing corruption problem infecting the scientific community, recently summarized the state of research on ocean “acidificaton” for the prestigious scientific journal Nature. He poignantly states that the lack of skepticism and an eager willingness to just accept the presumptions of others based upon their authoritative status (“groupthink”) may have “damage[d] the credibility of the ocean sciences”. And once scientific credibility is damaged, it becomes very difficult to earn that credibility back.
Cressey (2015) The state of the world’s seas is often painted as verging on catastrophe. But although some challenges are very real, others have been vastly overstated, researchers claim in a review paper. The team writes that scientists, journals and the media have fallen into a mode of groupthink that can damage the credibility of the ocean sciences. The controversial study exposes fault lines in the marine-science community. Carlos Duarte, a marine biologist at the University of Western Australia in Perth, and his colleagues say that gloomy media reports about ocean issues such as invasive species and coral die-offs are not always based on actual observations. It is not just journalists who are to blame, they maintain: the marine research community “may not have remained sufficiently sceptical” on the topic.
Scientists Find Higher CO2, Lowered pH Levels (‘Acidification’) Have Little To No Effect On Ocean-Dwelling Organisms
Scientists continue to construct experiments testing the effects of highly elevated CO2 (usually with volumes several times modern levels) on sea-living creatures. They routinely find that higher CO2 levels (and higher sea temperatures) have little to no effect on growth rates or survival for the species tested. In fact, it has been found in some cases that elevated CO2 benefits ocean-dwelling organisms, meaning that they thrive and prosper in these conditions. Obviously, these scientific studies wholly undermine the paradigm that envisions the long-term survival of the oceanic biosphere is jeopardized by rising anthropogenic CO2 emissions.
Uthicke et al., 2016 Near the vent site, the urchins experienced large daily variations in pH (> 1 unit) andpCO2 (> 2000 ppm) and average pH values (pHT 7.73) much below those expected under the most pessimistic future emission scenarios. Growth was measured over a 17-month period using tetracycline tagging of the calcareous feeding lanterns. Average-sized urchins grew more than twice as fast at the vent compared with those at an adjacent control site, and assumed larger sizes at the vent compared to the control site and two other sites at another reef near-by. … Thus, urchins did not only persist but actually ‘thrived’ under extreme CO2 conditions.
Vicente et al., 2016 The long-term exposure experiments revealed no effect on survival or growth rates of M. grandis to high pCO2 (1198 µatm), warmer temperatures (25.6°C), or combined high pCO2 with warmer temperature (1225 µatm, 25.7°C) treatments, indicating that M. grandis will continue to prosper under predicted increases in pCO2 and sea surface temperature.
Moore, 2016 If the forecasts of continued global warming are borne out, the oceans will also become warmer and will tend to outgas CO2, offsetting to some extent the small increased partial pressure that might otherwise occur. … An analysis of research on the effect of lower pH shows a net beneficial impact on the calcification, metabolism, growth, fertility, and survival of calcifying marine species when pH is lowered up to 0.3 units, which is beyond what is considered a plausible reduction during this century. … There is no evidence to support the claim that most calcifying marine species will become extinct owing to higher levels of CO2 in the atmosphere and lower pH in the oceans.
Hildebrandt et al., 2016 Elevated pCO2 did not directly affect grazing activities and body mass, suggesting that the copepods did not have additional energy demands for coping with acidification, neither during long-term exposure nor after immediate changes in pCO2. Shifts in seawater pH thus do not seem to challenge these copepod species.
Cross et al., 2016 A CO2 perturbation experiment was performed on the New Zealand terebratulide brachiopod Calloria inconspicua to investigate the effects of pH conditions predicted for 2050 and 2100 on the growth rate and ability to repair shell. Three treatments were used: an ambient pH control (pH 8.16), a mid-century scenario (pH 7.79), and an end-century scenario (pH 7.62). The ability to repair shell was not affected by acidified conditions with >80% of all damaged individuals at the start of the experiment completing shell repair after 12 weeks. Growth rates in undamaged individuals >3 mm in length were also not affected by lowered pH conditions.
Heinrich et al., 2016 In this study, we tested the effects of elevated CO2 on the foraging and shelter-seeking behaviours of the reef-dwelling epaulette shark, Hemiscyllium ocellatum. Juvenile sharks were exposed for 30 d to control CO2 (400 µatm) and two elevated CO2 treatments (615 and 910 µatm), consistent with medium- and high-end projections for ocean pCO2 by 2100. Contrary to the effects observed in teleosts and in some other sharks, behaviour of the epaulette shark was unaffected by elevated CO2.
Sunjin and Jetfelt, 2016 [A]n increasing number of studies show tolerance of fish to increased levels of carbon dioxide. … We investigated the possible effects of CO2 on behavioural lateralization, swimming activity, and prey and predator olfactory preferences, all behaviours where disturbances have previously been reported in other fish species after exposure to elevated CO2. Interestingly, we failed to detect effects of carbon dioxide for most behaviours investigated.
Schram et al., 2016 There were no significant temperature or pH effects on growth, net calcification, shell morphologies, or proximate body composition of snails. Our findings suggest that both gastropod species demonstrate resilience to initial exposure to temperature and pH changes predicted to occur over the next several hundred years globally and perhaps sooner along the WAP.
Brien et al., 2016 Corals were collected from reefs around Orpheus and Pelorus Islands on the Great Barrier Reef, Australia. They were then exposed to elevated pCO2 for 4 weeks with two CO2 treatments: intermediate (pCO2 648) and high (pCO2 1003) compared with a control (unmanipulated seawater) treatment (pCO2 358). Porites cylindrica growth did not vary among pCO2 treatments, regardless of the presence and type of competitors, nor was the growth of another hard coral species, Acropora cerealis, affected by pCO2 treatment.
Zhang et al., 2016 The present study investigated the physiological responses (ingestion, absorption rate and efficiency, respiration, and excretion) and scope for growth (SfG) of an intertidal scavenging gastropod, Nassarius festivus, to the combined effects of ocean acidification (pCO2 levels: 380, 950, and 1250 µatm), salinity (10 and 30 psu), and temperature (15 and 30°C) for 31 d. [E]levated pCO2 levels had no effects in isolation on all physiological parameters and only weak interactions with temperature and/or salinity for excretion and SfG. In conclusion, elevated pCO2will not affect the energy budget of adult N. festivus at the pCO2 level predicted to occur by the Intergovernmental Panel on Climate Change (IPCC) in the year 2300.
Wang et al., 2016 The pMENs results were in line with the null hypothesis that elevated pCO2/pH does not affect biogeochemistry processes. The number of nodes within the pMENs and the connectivity of the bacterial communities were similar, despite increased pCO2 concentrations. Our results indicate that elevated pCO2 did not significantly affect microbial community structure and succession in the Arctic Ocean, suggesting bacterioplankton community resilience to elevated pCO2.
Pančić et al., 2015 The effects of ocean acidification and increased temperature on physiology of six strains of the polar diatom Fragilariopsis cylindrusfrom Greenland were investigated. Experiments were performed under manipulated pH levels (8.0, 7.7, 7.4, and 7.1) and different temperatures (1, 5, and 8 °C) to simulate changes from present to plausible future levels. … By combining increased temperature and acidification, the two factors counterbalanced each other, and therefore no effect on the growth rates was found.
Wall et al., 2015 Cold-water corals are important habitat formers in deep-water ecosystems and at high latitudes. Ocean acidification and the resulting change in aragonite saturation are expected to affect these habitats and impact coral growth. Counter to expectations, the deep water coral Lophelia pertusa has been found to be able to sustain growth even in undersaturated conditions. … Skeletal morphology is highly variable but shows no distinctive differences between natural and low pH conditions. … We suggest that as long as the energy is available to sustain the up-regulation, i.e. individuals are well fed, there is no detrimental effect to the skeletal morphology.
Oceanic Microbes Routinely Endure Water Temperature Extremes That Exceed (Modeled) Future Warming Changes
According to climate models, sea surface temperatures are expected to rise dramatically during the next century due to the rise in anthropogenic CO2 emissions. Doblin and van Sebille (2016), however, point out that upper-ocean microbes routinely travel through (and thrive in) waters that vary in range by up to 10°C more than they do from one seasonal extreme to another (i.e., winter vs. summer), and thus the predicted warming of near surface ocean waters will not even be close to the extreme temperature variations these organism routinely endure.
Doblin and van Sebille, 2016 Here we show that upper-ocean microbes experience along-trajectory temperature variability up to 10 °C greater than seasonal fluctuations estimated in a static frame, and that this variability depends strongly on location. These findings demonstrate that drift in ocean currents can increase the thermal exposure of microbes and suggests that microbial populations with broad thermal tolerance will survive transport to distant regions of the ocean and invade new habitats.
[press release] The results of the study … show for the first time the range of temperatures that plankton travel through. In most locations, they endure temperature extremes that go beyond what is predicted by models of global warming.
Corals Naturally Adapt To Elevated CO2, Water Temperature; Their Long-Term Survival Is Not Threatened
Somehow corals were able to evolve and survive and thrive — avoiding extinction — during periods when atmospheric CO2 concentrations were several times higher than now, and when sea water temperatures were multiple degrees C warmer than now. And yet those advocating the ocean “acidification” narrative claim that corals are not longer able to adapt to the modern (tiny) temperature and CO2 changes that have occurred in recent decades. Scientists, meanwhile, have found that corals are quite resilient, and can adapt quickly to large environmental changes well beyond the range of recent and projected climatic conditions.
Prada et al., 2016 Our study suggests that populations of Orbicella species [corals] are capable of rebounding from reductions in population size under suitable conditions and that the effective population size of modern corals provides rich standing genetic variation for corals to adapt to climate change.
Matz et al., 2015 Heat tolerance in corals can be passed down the generations, suggesting that corals can adapt as the climate warms.
Georgiou et al., 2015 The FOCE experiment was designed to simulate the effects of CO2-driven acidification predicted to occur by the end of this century (scenario RCP4.5) while simultaneously maintaining the exposure of corals to natural variations in their environment under in situ conditions. Analyses of skeletal growth (measured from extension rates and skeletal density) showed no systematic differences between low-pH FOCE treatments (Δ pH=∼−0.05 to−0.25 units below ambient) and present day controls (ΔpH=0) for calcification rates or the pH of the calcifying fluid (pH cf) … [C]oral living in highly dynamic environments exert strong physiological controls on the carbonate chemistry of their calcifying fluid, implying a high degree of resilience to ocean acidification within the investigated ranges.
Corals Are A Net Source Of CO2, For They Release CO2 As They Grow And Thrive; ‘Acidification’ A Sign Of Healthy Corals
Scientists have found that higher “acidification” levels (lower pH) in the vicinity of coral communities are indication that the corals are thriving and growing. Why? Because corals produce their own “acidification” by releasing more CO2 than they absorb. They are a net source of CO2 to the atmosphere.
McGowan et al., 2016 Here we present by way of case study the first direct measurements of air-sea CO2 exchange over a coral reef made using the eddy covariance method. Research was conducted during the summer monsoon over a lagoonal platform reef in the southern Great Barrier Reef, Australia. Results show the reef flat to be a net source of CO2 to the atmosphere of similar magnitude as coastal lakes, while adjacent shallow and deep lagoons were net sinks as was the surrounding ocean. This heterogeneity in CO2 exchange with the atmosphere confirms need for spatially representative direct measurements of CO2 over coral reefs to accurately quantify their role in atmospheric carbon budgets.
Yeakel et al., 2015 Our results reveal that coral reefs undergo natural interannual events of rapid acidification due to shifts in reef biogeochemical processes that may be linked to offshore productivity and ultimately controlled by larger-scale climatic and oceanographic processes.
[press release] More acidic water may be a sign of healthy corals, says a new study, muddying the waters still further on our understanding of how coral reefs might react to climate change. … Andreas Andersson of the Scripps Institution of Oceanography in San Diego, California, and his colleagues carefully monitored a coral reef in Bermuda for five years, and found that spikes in acidity were linked to increased reef growth. … The researchers observed the chemistry of the water on the reef between 2007 and 2012. During that time, there were two sharp spikes in acidity – once in 2010 and again in 2011. The team found that coral growth itself made the water more acidic as the corals sucked alkaline carbonate out of the water to build their skeletons. The corals also ate more food during these high-activity periods and pumped more CO2 into the water, increasing acidity further.
More Than 90% Of Ocean pH Changes (‘Acidification’) Caused By Natural Variability, Not Anthropogenic CO2
Finally, the assumption that changes in the oceans’ pH levels are primarily caused by humans is just that: a non-confirmed assumption. As Duarte et al. (2015) conclude, there is “no robust evidence for realized severe disruptions of marine socioecological links from ocean acidification to anthropogenic CO2”. Possibly the only people who still “believe” in the paradigm are those who are inclined to accept doomsday scenarios and those who are being financially compensated to keep them going.





Monterey Bay Aquarium – pH of incoming ocean water, 1996-2009.
http://sanctuarymonitoring.org/regional_docs/monitoring_projects/100240_167.pdf
Large variation with NO discernible trend either up or down. No smoking gun there.
It is always the same: you have found 2 or 3 papers who mention the term “acification” and claim little effect. so you generalise to “acification does not exist”.
This is anti science and contra-factual.
a single day at a university would show you, how things work in the real world. Real scientists have read these papers. They are included in their opinions. facts matter.
It’s a little worse than that, sod. There are not “2 or 3” papers referenced here. There are 23. Close, though. And these are just samples of what’s available in the scientific literature.
Here are a few others that show that varying pCO2 by 285 ppm to 4,568 (pH range 8.1 to 7.1) over a 4-week period did not affect the growth and calcification of corals.
—–
http://link.springer.com/article/10.1007%2Fs00338-014-1241-3
This study investigated the response of the gorgonian coral Eunicea fusca to a range of CO2 concentrations from 285 to 4,568 ppm (pH range 8.1–7.1) over a 4-week period. Gorgonian growth and calcification were measured at each level of CO2 as linear extension rate and percent change in buoyant weight and calcein incorporation in individual sclerites, respectively. In general, growth and calcification did not stop in any of the concentrations of pCO2… These results highlight the susceptibility of the gorgonian coral E. fusca to elevated levels of carbon dioxide but suggest that E. fusca could still survive well in mid-term ocean acidification conditions expected by the end of this century, which provides important information on the effects of ocean acidification on the dynamics of coral reef communities.
—–
And another that varied seawater CO2 to levels of 430, 907, 1,865, and 3,247 ppm over the course of 6 months, but found corals were “largely unaffected” by each incremental CO2 change.
—–
http://www.biogeosciences.net/11/1581/2014/bg-11-1581-2014.html
Calcifying foraminifera are expected to be endangered by ocean acidification; however, the response of a complete community kept in natural sediment and over multiple generations under controlled laboratory conditions has not been constrained to date. During 6 months of incubation, foraminiferal assemblages were kept and treated in natural sediment with pCO2-enriched seawater of 430, 907, 1865 and 3247 [ppm] pCO2. The fauna was dominated by Ammonia aomoriensis and Elphidium species, whereas agglutinated species were rare. After 6 months of incubation, pore water alkalinity was much higher in comparison to the overlying seawater. Consequently, the saturation state of Ωcalc was much higher in the sediment than in the water column in nearly all pCO2 treatments and remained close to saturation. As a result, the life cycle (population density, growth and reproduction) of living assemblages varied markedly during the experimental period, but was largely unaffected by the [ppm] pCO2 treatments applied.
—–
And by the way, sod, it’s not “acification”, it’s acidification. I’d assume you’d want to use the proper scientific terminology.
“2 or 3″…”23″…it’s easy to see how a functionally-illiterate progressive (but I repeat myself) could mistake one for the other.
Common Core Arithmetic.
Kenneth Richard,
I believe the correct term is becoming less base, the term acidification was first was coined in 2003 by climate scientist Ken Caldeira to frighten half-wits like Sod.
“THE NAME
Why is this phenomenon called “ocean acidification”, even if our oceans will never actually
become acidic (pH < 7)? Acidification is a process: the decrease in pH (increase in hydrogen
ions and acidity). The word “acidification” refers
to lowering pH from any starting point to any
end point on the pH scale. This terminology can
be compared to the one used for temperature:
if the temperature of the air goes from -20 to -10,
https://www.oceanfdn.org/sites/default/files/ocean-acidification_ScientificItems.pdf
i
Can you, perhaps, kindly tell us where you obtain your information? Is it published? Can you, please, cite references etc as scientists do every day.
Perhaps “English as a Second Language” would be a course to take in the New Year.
You are at the moment giving a very bad impression of German Education,which I hope is erroneous.
concerned frequent reader of this worldwide blog.
Perhaps “English as a Second Language” would be a course to take in the New Year.2
sounds good. We can also switch the debate to German and one of you folks translates. Then you can really show off your superior language skills!
Don’t think it would matter what language, your posts would still be nothing but empty brain-washed parroted propaganda.
“a single day at a university would show you, how things work in the real world. Real scientists have read these papers. They are included in their opinions. facts matter.”
This is probably the most BIZARRE sentences even sop has ever written..
Single day at a university would show you how things work in real world ???????
Seriously ?????
…. included in their opinions… facts matter ???????
roflmao..
I’m sure he never actually reads anything he writes.
“Single day at a university would show you how things work in real world ??????? ”
yes it would. This methodology (simply making a list of papers with a certain keyword) would be greeted with utter disbelieve.
People would point out the majority of papers that disagree. and they would point out flaws in the papers he cites. But mostly the problem is, that these papers do not contradict the stuff they are supposed to contradict.
Your utter idiocy at thinking that University is the real world, is clownishly moronic to say the least.
It is obvious that you are totally unable to point out any flaws in the papers he cites. I doubt you have even read one of them, let alone understood what is being said.
Kenneth does just cite them, he tells you what is in them, because he knows you are incapable of doing the leg work yourself.
“This methodology (simply making a list of papers with a certain keyword) would be greeted with utter disbelieve.” – sod (still a socialist donkey)
Yes, after all, who wants papers that actually address the topics of interest.
That is how you find papers, sod.
“A keyword is a key to information. Keywords point researchers to relevant papers—…”
http://www.editage.com/insights/why-do-journals-ask-for-keywords
How much are you getting paid by Soros to spew your nonsense?
Title of post-truth novel: ‘Sod, his life and his opinions’.
Sod,you are the only one to mention “acification”.
What is acification?
Sod,
your comment is really bad.
No specific objection to ANY of the papers Ken posted here.
What is the cause of your habitual failure to make worthy detailed opposing comments?
he/she/it/whatever seems to be slowing down lately. Probably needs a software upgrade and a reboot.
“Probably needs a software upgrade and a reboot.”
No, just a steel-cap boot… right as he leaves.
“A single day at a university would show you, how things work in the real world”
That’s the best laugh you’ve provided all year!!! A university is the VERY LAST PLACE where you would find people with experience of the real world…
So he *IS* in a home for the specially abled…
Earth has 310 million cubic miles of ocean with pH range from alkaline pH from 6.8 to 8.4 depending on temperature, depth, fresh water inflows. Earth’s oceans are lined with basalt and other Calcium Carbonate rocks. Carbonic acid is weak, with a half life of 20 milliseconds outside of a laboratory beaker.
“Volcanic CO2” by Timothy Casey at Geology-1011
Another great review article. Things certainly look different when based on experimental and observational data. So much for assumptions and models. Thank you Ken – this is the best on-going series related to global warming because it is based on peer-reviewed papers by qualified scientists. Well referenced – well done.
Stay tuned…
Next Monday is going to be big in terms of “references” from the scientific literature.
The work you have done here is ground breaking. You deserve the highest praise. In fact, there is so much it is going to take me months to digest the treasure trove. +1000.
Considering that when hard corals first appear in the fossil record, atmospheric CO2 was 5x higher than it is today, it should be no surprise that these organisms would not be adversely impacted by increased CO2. They were “raised on it”.
Kenneth Richards,
thank you for your efforts to put the science out on Pierre’s fine blog.
Kenneth:
You do realize, I hope, that if you can disprove ocean acidification science, you will instantly be world famous, win the Nobel Prize in Chemistry, and get all the money and women (or guys) that you what.
So why are you fooling around on this dinky blog?
Why not publish your world-shattering results in Science or Nature???
David
To “disprove” the “science” of the position that anthropogenic CO2 emissions have directly caused the pH levels of the global oceans to “fall” by 0.1 since the the 1800s, and that this human-caused phenomenon is currently having a deleterious effect on oceanic organisms to the exclusion of all other potential causal variables (if this deleterious effect is indeed happening) would imply that the human CO2–> pH-lowering of 0.1 cause-effect relationship has already been scientifically proven and/or confirmed using observational evidence and controls, ruling out all other potential causal variables. This would imply that such a scientific experiment has already been conducted. It hasn’t. These are modeled assumptions.
You are presumptuously claiming that your beliefs are proven scientific fact. They are not.
Kenneth, notice how you quickly changed the subject.
I know why you do that.
Um, no, I didn’t change the subject. Assuming you agree with the claim that human CO2 emissions have directly caused a pH lowering of -0.1 in the last 150-200 years, you suggested I need to “disprove” this claim. Disproving your claim implies that it has already been scientifically proven as true, that it is a verifiable fact. Can you point to the experimental scientific evidence that shows human CO2 emissions cause oceanic pH levels to decrease on centennial scales, but that the natural factors and variations which contribute to + or – 0.2 to 0.5 decadal-scale changes in pH do not have any influence whatsoever on detectable centennial-scale trends? In other words, provide the physical, observational evidence that shows natural variability contributes nothing to centennial-scale trends.
But speaking of publishing in Nature and Science, it looks as though this has already been done.
http://www.nature.com/news/ocean-calamities-oversold-say-researchers-1.16714?WT.mc_id=TWT_NatureNews
The state of the world’s seas is often painted as verging on catastrophe. But although some challenges are very real, others have been vastly overstated, researchers claim in a review paper. The team writes that scientists, journals and the media have fallen into a mode of groupthink that can damage the credibility of the ocean sciences. The controversial study exposes fault lines in the marine-science community. Carlos Duarte, a marine biologist at the University of Western Australia in Perth, and his colleagues say that gloomy media reports about ocean issues such as invasive species and coral die-offs are not always based on actual observations. It is not just journalists who are to blame, they maintain: the marine research community “may not have remained sufficiently sceptical” on the topic.
http://www.nature.com/ismej/journal/v6/n9/full/ismej201219a.html
We found that pH did not have a significant impact on the composition of associated microbial communities in both coral species. In contrast to some earlier studies, we found that corals present at the lower pH sites exhibited only minor physiological changes and no microbial pathogens were detected. Together, these results provide new insights into the impact of ocean acidification on the coral holobiont.
http://www.nature.com/nclimate/journal/v2/n8/full/nclimate1473.html
Using a model of pH regulation combined with abiotic calcification, we show that the enhanced kinetics of calcification owing to higher temperatures has the potential to counter the effects of ocean acidification.
http://www.sciencemag.org/content/344/6186/895.abstract
Reef corals are highly sensitive to heat, yet populations resistant to climate change have recently been identified. To determine the mechanisms of temperature tolerance, we reciprocally transplanted corals between reef sites experiencing distinct temperature regimes and tested subsequent physiological and gene expression profiles. Local acclimatization and fixed effects, such as adaptation, contributed about equally to heat tolerance and are reflected in patterns of gene expression. In less than 2 years, acclimatization achieves the same heat tolerance that we would expect from strong natural selection over many generations for these long-lived organisms. Our results show both short-term acclimatory and longer-term adaptive acquisition of climate resistance. Adding these adaptive abilities to ecosystem models is likely to slow predictions of demise for coral reef ecosystems.
May Be you will get a “co-nobel” like Mann did for poiting out those papers to the Nobel Committee? 🙂
“that if you can disprove ocean acidification science”
Buffering of multi-trillion of tonnes of alkaline rock surrounding ocean basic… huge masses of calcite and carbonate material in the oceans themselves.
Nearly every river that flows into the oceans is acidic.
Over millions and millions of years, the oceans have absorbed that acidic water with no change in pH.
If you really think a tiny change in atmospheric CO2, of which a reasonable amount actually came from the oceans anyway, is going to affect the ocean pH… you have to be on some sort of hallucinogenic drug or have undergone some very deep brain-washing to the extent that there is basically no brain left.
AndyG55 30. December 2016 at 6:40 AM,
Is that why all the white cliffs the world over have disappeared?
/sarcoff
David Appell,
first you have to measure the sea’s ph accurately-
$2 Million Dollar Prize to Benefit Ocean Research – Ocean Home
http://www.oceanhomemag.com/2-Million-Dollar-Prize-Aiding-Ocean-Research/
They are offering two $1 million dollar prizes to those who develop an affordable and accurate tool to measure pH levels in the ocean,
Wow, I thought that from everyone saying there had been a decimal place change in pH over x years since “CO2 was invent”, they must have had equipment capable of measuring ocean pH to 1 or 2 dp, like 50-100 years ago !!
Now I’m so disheartened to know that they don’t even have that equipment in the present. 😉
Ocean pH is so notoriously difficult to measure that I am amazed that such an “expert” as DoA Appell is unaware of the fact – NOT.
When avg., ocean pH ranges from 7.3-8.2, claims of being able to measure a change of a tenth of a pH unit or less in seawater needs to be taken with more than a grain of sea salt.
http://www.cambridge.org/resources/0521538432/1488_218437.pdf
Near undersea hydrothermal vents, where the pH of the water actually IS acidic, you will find a number of shellfish, like this one whose shell is clearly NOT dissolving.
http://files.campus.edublogs.org/blog.nus.edu.sg/dist/9/814/files/2010/04/calyptogena.jpg
Why not publish some actual data that shows there is an neutralisation of the alkaline oceans, actually happening.
Its just another baseless, and erroneous ass-umption.
Gosh, David with that big PHD sticking out of his back pocket,fails to do what he tells Kenneth to do.
Selective quotes,
“You do realize, I hope, that if you can disprove ocean acidification science, you will instantly be world famous, win the Nobel Prize in Chemistry, and get all the money and women (or guys) that you what.”
“Why not publish your world-shattering results in Science or Nature???”
But Kenneth easily took down this warmist butterball,with this obvious statement,selected quote:
“This would imply that such a scientific experiment has already been conducted. It hasn’t. These are modeled assumptions.
You are presumptuously claiming that your beliefs are proven scientific fact. They are not.”
David, where is that science paper,you imply exist…………………..!!!!!!!
David why can’t you drop your love affair with a trace gas,get back to scientific reality?
Sun: If you don’t know about papers on ocean acidification, it’s only because you keep your head buried in the sand. It’s not my responsibility to tutor you on the basics.
“Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean,” US National Research Council, National Academy of Sciences (2010).
http://dels.nas.edu/Report/Ocean-Acidification-National-Strategy/12904
The very first paragraph from your link,
“Excess carbon dioxide in the atmosphere—in addition to contributing to climate change—is absorbed by the ocean, making sea water more acidic and leading to a suite of changes in ocean chemistry. Preliminary evidence suggests ocean acidification will have negative effects on corals, shellfish, and other marine life, with wide-ranging consequences for ecosystems, fisheries, and tourism. This report, requested by Congress, reviews the current state of knowledge and identifies gaps in understanding, and provides scientific advice to help guide the national ocean acidification research program.”
This is an extremely stupid paragraph,supposedly written by well educated people,but it is still stupid.
It is also dishonest and misleading,since there are a lot of papers that doesn’t support the claims brought up in the paragraph.
Kenneth post just a sampling of papers showing the very opposite,which is why we are laughing at you. You have already indicated that you are going to ignore the papers Ken posted because you are too deep into the CO2 delusion.
The very first paragraph from your link,
“Excess carbon dioxide in the atmosphere—in addition to contributing to climate change—is absorbed by the ocean, making sea water more acidic and leading to a suite of changes in ocean chemistry. Preliminary evidence suggests ocean acidification will have negative effects on corals, shellfish, and other marine life, with wide-ranging consequences for ecosystems, fisheries, and tourism. This report, requested by Congress, reviews the current state of knowledge and identifies gaps in understanding, and provides scientific advice to help guide the national ocean acidification research program.”
This is an extremely stupid paragraph,supposedly written by well educated people,but it is still stupid.
It is also dishonest and misleading,since there are a lot of papers that doesn’t support the claims brought up in the paragraph.
Kenneth post just a sampling of papers showing the very opposite,which is why we are laughing at you. You have already indicated that you are going to ignore the papers Ken posted because you are too deep into the CO2 delusion.
I did my own analysis of how much human Co2 that becomes carbonic acid in the oceans amounts to. Answer: on an annual basis: one molecule of carbonic acid traceable to human Co2 absorbed by the ocean to 107,000,000,000 (107Billion) parts seawater.
That’s the equivalent of 1 eye-drop of acid in 40 swimming pools of water 20m x 6m x 2m in volume.
Any high school graduate could do the same analysis.
Oh, NOOOS.
We’re on the very brink of DOOM!
:-0)
Compare that to the millions of years of water from literally thousands of rivers, many of which can have pH down to 5.5, flowing into the ocean..
And the ocean pH hasn’t budged, it remains stubbornly alkaline around pH 8.1 +/- a bit.
“That’s the equivalent of 1 eye-drop of acid in 40 swimming pools”
And carbonic acid from the combination of CO2 with water is a rather weak acid.
It is used by crustaceans to build into carbonates for shells etc.
If you look in a decent chemical dictionary, you will find a description something like this..
“Carbonic acid (H2C03). The hypothetical acid of carbon dioxide and water. Carbonic acid exists only in the form of its salts (carbonates), acid salts (hydrogen carbonates), amines (carbamic acid), and acid chlorides (carbonyl chloride). ”
As indicated by the names, the last two require nitrogen, a chlorine respectively.
Show your work.
Better yet, publish it in a real journal, show all the world’s scientists they are wrong, and win a Nobel Prize in Chemistry.
Here’s that big chance your mommy always readied you for….
Ha ha ha,
David Appell,sure knows how to prove that he is all wind and piss. It is clear YOU have ZERO counterpoints to make to the post,which is why some of us are laughing at your stupid comments, “show your work”,when Kenneth did just that by posting many PUBLISHED papers on it. Heck Ken even BOLDED some of it for the reading challenged,yet YOU still missed it!!!
You are a lost boy,David.
Poor David.. the real reactions of CO2 in heavily buffered seawater have been known for ages.
It is those claiming a tiny increase in atmospheric CO2 can make any difference to ocean pH who are going against that known chemistry.
I’ll give you that quick breakdown of the FACTS again
Please do try to learn at least some tiny amount this time.
“Carbonic acid (H2C03). The hypothetical acid of carbon dioxide and water. Carbonic acid exists only in the form of its salts (carbonates), acid salts (hydrogen carbonates), amines (carbamic acid), and acid chlorides (carbonyl chloride). ”
” Carbonic acid exists only in the form of its salts (carbonates), acid salts (hydrogen carbonates), amines (carbamic acid), and acid chlorides (carbonyl chloride). ”
….and in the water on David Appell’s hypothetical brain.
The whole Global Warming hoax has nothing to do with science and everything to do with politics. The Globalsts pushing this nonsense are being rejected wherever the public has not been brainwashed.
The acidification scam scares people like sod and appell who do not understand that our oceans remain stubbornly alkaline in spite of what corrupt “Scientists” may say.
The “Debate is Over” and the Warminsts lost. All that remains are scientists feeding at government troughs and useful idiots such as the trolls who still show up here
GC: Have you found that missing 150 W/m2 yet?
I didn’t think so.
Perhaps that should be your first priority, lest the Earth collapse into a very deep freeze.
yawn.. can we find an IMAGINARY 150w/m².
Eat the correct mushrooms , I’m sure you will find it somewhere in you feeble imagination.
Andy,
Here is the link that launched the deranged Appell into an alternate reality:
https://diggingintheclay.wordpress.com/2013/03/07/arrhenius-revisited/
He just can’t accept the idea that Arrhenius was wrong. Any comments?
In the first paper of this entry (Wei et al., 2015), a visual examination of the first illustation which show overlain graphs of time vs Temp and time vs PH, there appears to be a 10 year offset of the data. Ocean temperature increase and decreases precede PH changes by 10 years. The graphs show that decreases and increases of oceanic temperature are followed by a ~10-15 year lag with respective increases and decreases in PH.
We know that Carbonate is more stable in warm water, and less stable in cold water, also that there is a lag in the mixing of oceanic waters both at he surface and at depth.
Yes, another problem for the paradigm is that as ocean waters warm, they release more of their vast stores of CO2. As they cool, they retain more. So “acidification” is more likely to occur when waters are cooling than warming.
This is reflected in the rate change of CO2 increases. It’s much higher during El Nino years (’97-’98, ’15-’16) than during La Nina years (1999-’00, 2008).
Excellent debunking of the ocean acidification scam.
For the CAGW axis, opening a second front on ocean acidification will prove disastrous, as Pierre makes clear.
So you think — tell me if I’m right here — that a body of water that absorbs CO2 does not have an increasea in carbonic acid and that its acidity does not increase? And your science?
Do you know how absolutely dimwitted that is? How purely stupid you are?
David,fails to realize that the Ocean waters already has 99% of the free CO2 in it,a dribble more added to it surely can’t make that much difference.
He also fails to realize that most rivers of the world,that empty’s into the Oceans is acidic,so is the rain. Yet the Ocean waters are still strongly ALKALINE after a Billion years.
“How purely stupid you are?”
Appell’s ignorance on display again.
And decent chemistry book will show you a did more about “carbonic acid”
“Carbonic acid (H2C03). The hypothetical acid of carbon dioxide and water. Carbonic acid exists only in the form of its salts (carbonates), acid salts (hydrogen carbonates), amines (carbamic acid), and acid chlorides (carbonyl chloride). ”
Crustaceans use the combination of CO2 and H2O to make their shells.
Appell ignores the chemistry, just like he ignores every other actual fact that doesn’t support his brain-washed AGW scam.
Sea water is stubbornly alkaline and the increase in atmospheric CO2 will not change that. You should not use the word “Acid” until the pH falls below 7.
You don’t seem to have a head for figures but I will try again. The amount of CO2 in the atmosphere is ~3,000 Giga-tonnes while the amount of CO2 dissolved in the oceans is more than ten times greater. Humans add only ~30 Giga-tonnes per year.
The more rabid Alarmists claim that ocean pH will fall to 7.8 by 2100 but that is still ALKALINE. I sincerely doubt this will happen and even if it did it would not matter. I have grown salmonids in water with a pH of 6.5 without distressing the fish.
CO2 has little effect on ocean pH owing to “Buffering”:
http://nsgl.gso.uri.edu/ncu/ncue90001/ncue90001chap3.pdf
Sea water is stubbornly alkaline and the increase in atmospheric CO2 will not change that. You should not use the word “Acid” until the pH falls below 7.
You don’t seem to have a head for figures but I will try again. The amount of CO2 in the atmosphere is ~3,000 Giga-tonnes while the amount of CO2 dissolved in the oceans is more than ten times greater. Humans add only ~30 Giga-tonnes per year.
The more rabid Alarmists claim that ocean pH will fall to 7.8 by 2100 but that is still ALKALINE. I sincerely doubt this will happen and even if it did it would not matter. I have grown salmonids in water with a pH of 6.5 without distressing the fish.
CO2 has little effect on ocean pH owing to “Buffering”:
http://nsgl.gso.uri.edu/ncu/ncue90001/ncue90001chap3.pdf
David, was given a smackdown by Bob Tisdale,exposing Davids lies:
Comment From Bob Tisdale
” Bob Tisdale says:
February 1, 2013 at 2:56 pm
davidappell says: “Tisdale offers no reason for ocean warming, except if you buy his book. (=suckers.)”
Another untruth from davidappell. Falsehoods are a staple of proponents of manmade global warming like you, David. Everyone knows the vast majority of the content of my book “Who Turned on the Heat?” has been discussed in dozens of blog posts over the past 4 years. In addition, I’ve produced two YouTube presentations for the WUWT-TV special. See here:”
Rest of it here:
https://stevengoddard.wordpress.com/2013/02/01/comment-from-bob-tisdale/
Nice.
Thank you.
You welcome.
Not long after that he got banned,in part because I drove him crazy.He made a meltdown comment naming ME and Tony as the two he was so upset about. Tony them lifted that comment to a Blog post to show how juvenile David was in his rant.
He didn’t last long after that.
You wouldn’t happen to have that link, would you? It would be a handy cudgel to use on him when he gets out of hand.
Here’s one from Lubos Motl responding to a D.A. comment that got units mixed up.
http://motls.blogspot.com/2012/07/have-muller-or-watts-transformed-agw.html#comment-605585774
Tried to find it,but not successful. It is in the OLD Blog page,somewhere.
OK.
@yonason,
Trust Lubos to put Appell in his place…….he does not suffer fools gladly.
Lubos booted me off his site for complaining about his acceptance of adverts supporting the “Nicholas School of the Environment”.
I haven’t been booted off yet, at least I don’t think so. I haven’t been back to check his response to my last post. =(
Thanks.
So you think – tell me if I’m right here – that the same phyla of calcofied animals like corals which evolved, thrived and spread in the Cambrian with atmospheric CO2 at 5000-10000 ppm, are now threatened by increases of CO2 from 400 by another one or two hundred ppm?
And the problem with your ocean “acidification” second front is that you can’t hide your palaeo denial behind a “dim sun”. It doesn’t work here. The only thing that’s dim is you.
Canadian Water Quality
Guidelines for the Protection
of Aquatic Life
pH
(Marine)
“The pH of marine waters is usually quite stable (between
7.5 and 8.5 worldwide) and is similar to that of estuarine
waters because of the buffering capacity provided by the
abundance of strong basic cations such as sodium,
potassium, and calcium and of weak acid anions such as
carbonates and borates (Wetzel 1983). Higher pHs are
usually found in near-surface waters because of solar
radiation Biological Effects”
[…] https://notrickszone.com/2016/12/29/the-ocean-acidification-narrative-collapses-under-the-weight-of-n… […]