Earth’s atmosphere is made of 78% nitrogen (N2) and 21% oxygen (O2). The “consensus” view is N2 and O2 are not greenhouse gases (GHGs) and don’t absorb infrared radiation (IR). But scientists have been saying N2 absorbs and radiates IR since 1944 and more recent (2012, 2016) studies have found N2 and O2 are “radiatively important” greenhouse gases with IR temperature absorption capacities similar to CO2.
It’s been known for 75 years that nitrogen – the Earth’s most prevalent atmospheric gas – absorbs and “strongly” radiates infrared energy (Stebbins et al., 1944)

Image Source: Stebbins et al., 1944
Methane (CH4) is thought to be an 84 times more potent greenhouse gas than CO2.

Image Source: Environmental Defense Fund
Nitrogen, oxygen are “natural greenhouse gases”
Scientists (Höpfner et al., 2012) publishing in Geophysical Research Letters dispute the “common perception” that nitrogen and oxygen – accounting for 78% and 21% of the Earth’s atmospheric gases – do not contribute signficantly to the Earth’s greenhouse effect.
They assert N2 and O2 are “radiatively important” “natural greenhouse gases” primarily because their concentration is “about 2000 (550) times higher than that of CO2 and about 4.4 × 105 (1.2 × 105) times more abundant than CH4.”
Nitrogen, oxygen combined are more potent GHGs than methane
The atmospheric abundance of N2 and O2 compensates for their relatively weaker IR function (when directly compared to CH4).
For example, “the natural greenhouse effect of N2 and O2 would be larger than that of CH4 by a factor of 1.3” when considering their combined isolated GHE influence.
Further, the reduction in the atmosphere’s infrared transmission amounts to 25.7% for N2, 14.2% for O2, and only 6.9% for CH4.
Nitrogen’s greenhouse gas influence also rivals CO2’s
Höpfner and colleagues also suggest N2 reduces outgoing longwave radiation (OLR) by 4.6 W/m² compared to CO2’s 5.1 W/m² when assesing their solo absorption capacity. This would appear to be a rather minor difference.
If the number of N2 molecules in the atmosphere were hypothetically doubled, it would produce a 12 W/m² longwave greenhouse effect forcing.
Doubling CO2 from 280 ppm to 560 ppm only yields a 3.7 W/m² radiative forcing.
The authors reject the “view that the radiative forcing of N2 increase operates only indirectly by broadening the absorption lines of other gases.” Instead, N2 has a “direct impact” (as well as an indirect impact) within greenhouse effect forcing.
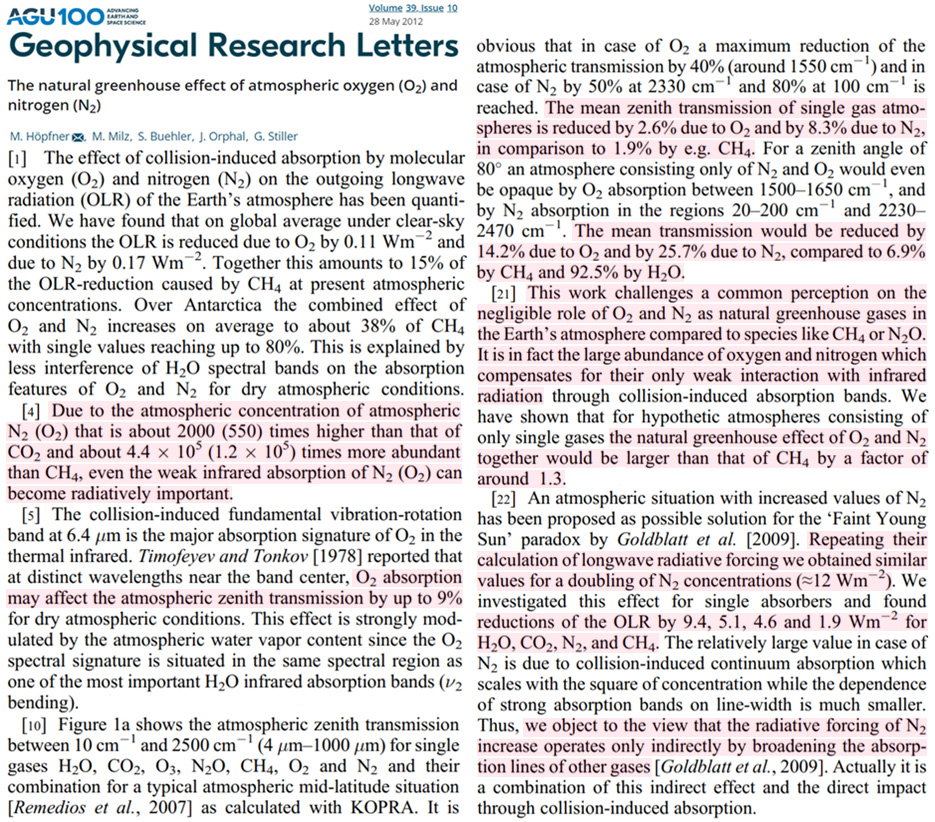
Image Source: Höpfner et al., 2012
Experiment: nitrogen, oxygen absorb IR to about the same limiting temperature as CO2
A real-world experiment (Allmendinger, 2016) assessing the efficacy of CO2’s IR-absorption temperature capacity relative to air (N2, O2) and Argon (Ar) further establishes CO2 is not the “special” GHG it is commonly thought to be.
Twin styrofoam Saran-wrap-sealed tubes exposed to sunlight were used, one with pure (1,000,000 ppm) CO2 and the other with air (N2, O2) and/or Ar.
The results were admittedly “surprising” given expectations CO2 would operate as a radiatively distinct GHG.
The tube absorbing IR with N2 and O2 (air) and Ar warmed to a temperature limit quite similar to (55°C to 58°C) the temperature limit in the 100% CO2 tube (58°C).
There was no remarkable or “special” heat absorption capacity for CO2 relative to air observed. And Argon – not considered a greenhouse gas – absorbed IR to the same temperature limit as CO2. With a concentration of 9300 ppm, Ar is the third-most abundant gas in the Earth’s atmosphere.
Because there is so little to distinguish CO2 from the most abundant gas molecules in Earth’s atmosphere, Dr. Allmendinger assesses “a significant efect of carbon-dioxide on the direct sunlight absorption can already be exluded.”
Further, “the greenhouse theory has to be questioned.”






[…] https://notrickszone.com/2020/02/10/scientists-oxygen-nitrogen-radiatively-important-greenhouse-gase… […]
I think R. W. Wood explained it best in his Note on the Theory of the Greenhouse:
“Is it therefore necessary to pay attention to trapped radiation in deducing the temperature of a planet as affected by its atmosphere? The solar rays penetrate the atmosphere, warm the ground which in turn warms the atmosphere by contact and by convection currents. The heat received is thus stored up in the atmosphere, remaining there on account of the very low radiating power of a gas. It seems to me very doubtful if the atmosphere is warmed to any great extent by absorbing the radiation from the ground, even under the most favourable conditions.”
you left out the best bit, he was an expert in the field of ultraviolet radiation.
Allmendingers experiment is the worst you can do.
It is not possible to detect the effect of absorbing/radiation in that experiment, and he should know that.
The title of the Allmendinger paper is: “The thermal behavior of gases……” I think he can show very well the difference in the temperature between various gases under the influence of sunlight and IR radiation.
The whole concept of “greenhouse gas” is fundamentally flawed. It is based on the average surface temperature of the earth v the black body OLR from some some fictitious surface in the atmosphere.
The surface temperature of the globe is fundamentally a function of the wide distribution and connectedness of surface water. The atmosphere plays its role in driving ocean currents that distribute the excess heat collected in the tropical oceans and transport to higher latitudes. The ocean surface cannot exceed much over 30C because the rate of evaporation rises so strongly above that level that the resulting cloud reflects insolation. At the low end, the water surface cannot go below -2C because ice forms on the surface that insulates the water from further heat loss. The global average sea surface temperature of 16C is smack in the middle of these two extremes. It simply cannot vary much from this average value due to the controls on the extremes.
Atmospheric water vapour and outgoing long wave radiation are positively correlated – dead opposite of what the fairy tale states:
https://1drv.ms/b/s!Aq1iAj8Yo7jNg1uzA-KKFEvD5BzX
I am a little confused.The experiment and this explanation do not seem to agree. Are they both in error or am I missing something.
How does the CO2 analyzer work?
Air is slowly pumped through a small cylindrical cell with flat windows on both ends. Infrared light is transmitted through one window, through the cell, through the second window, and is measured by a detector that is sensitive to infrared radiation. In the atmosphere carbon dioxide absorbs infrared radiation, contributing to warming of the earth surface. Also in the cell CO2 absorbs infrared light. More CO2 in the cell causes more absorption, leaving less light to hit the detector. We turn the detector signal, which is registered in volts, into a measure of the amount of CO2 in the cell through extensive and automated (always ongoing) calibration procedures
If CO2 absorbs infrared radiation energy it would contribute to a warming of said CO2. How will it then contribute to the warming of the surface, which just has lost this energy?
Something is wrong with that statement.
I may be wrong, but it seems Allmender’s paper does not mention varying pressure, maybe due to the lightweight materials, presumably done at normal altitudes.
Remembering Nikolov and Zeller’s
https://www.researchgate.net/publication/317570648_New_Insights_on_the_Physical_Nature_of_the_Atmospheric_Greenhouse_Effect_Deduced_from_an_Empirical_Planetary_Temperature_Model
it sure seems like a confirmation….
We must act at once to eliminate all oxygen and nitrogen from the atmosphere.
A very slight correction, the Stebbins paper was published in January, 1945 https://ui.adsabs.harvard.edu/abs/1945ApJ…101…39S/abstract.
[…] Gains Nothing from Anecdotes Con Ed’s Annual Report on CO2, CH4 (Methane) and SF6 Scientists: Oxygen & Nitrogen ‘Radiatively Important’ Greenhouse Gases Exposing Climate Alarmist Michael Mann’s Criminal Record Guess Who’s Leading The World In CO2 […]
N2 and O2 do have IR spectra and they are measured (with their respective temperatures) with modern Raman IR spectrometers. To end this nonsense we need to take a deeper look at the physics and chemistry of the atom and what the modern laser-based Raman IR spectrometer is revealing.
Raman spectrometers (RS) can measure the quantum predicted positions of all the so-called ‘non-IR’ emission spectra (O2 and N2’s single spectra; the single spectra of CO2 and 2 of CH4’s; H2Os modes are both IR and Raman active spectra) and it can exquisitely measure – via the Boltzman constant – the temperature of the molecules. RS is a new type of thermometer and is the known complement instrument to the GHG deriving instrument, the IR spectrometer. RS is telling us all the gases absorb and emit IR radiation – as they should, how did we ever think they didn’t?! All matter with a temperature radiates IR; that’s the fundamentals.
Raman spectroscopy – I am saying – totally contradicts both radiation and greenhouse theories whereby the ‘Raman active’ modes are assumed not to radiate IR at all. Raman spectrometers are widely used and are becoming the instrument of choice on solar system space probes. Here is one that had measured the concentrations and presence of the gases of Venus. https://www.lpi.usra.edu/vexag/jan_2007/thursday/wang230.pdf
Read my working papers. I am going to try and publish, feel free to help. ‘Quantum Mechanics and Raman Spectroscopy Refute Greenhouse Theory’ http://vixra.org/abs/1811.0498 and ‘The Greenhouse Gases and Infrared Radiation Misconceived by Thermoelectric Transducers’ http://vixra.org/abs/1811.0499