A new balloon experiment (inadvertently) demonstrates just how weak and inconsequential CO2’s warming effect is.
Levendis et al. (2020) tried using an experiment with air-filled (99% nitrogen and oxygen) versus CO2-filled (100% CO2) balloons to demonstrate a consequential warming effect for CO2. But their study may have demonstrated the opposite.
In the experiment a heat source was applied and then removed (shut off) to 3 different-sized balloons with 100% (1,000,000 ppm) CO2 in them. They compared the rate of cooling for these CO2-filled balloons to the cooling rate for a balloon filled with regular room air that has ~790,000 ppm nitrogen (N2), ~210,000 ppm oxygen (O2), ~9,000 ppm Argon (Ar), and ~500 ppm CO2.
The results revealed the temperature inside the 1,000,000 ppm CO2 balloons cooled just ~1.3°C (0.5 to 2.0°C) more slowly after 6½ minutes than the balloon with ambient air with ~500 ppm CO2. Put another way, raising CO2 concentrations from 500 ppm to 1,000,000 ppm only leads to a warming (reduced cooling) effect of 1.3°C.
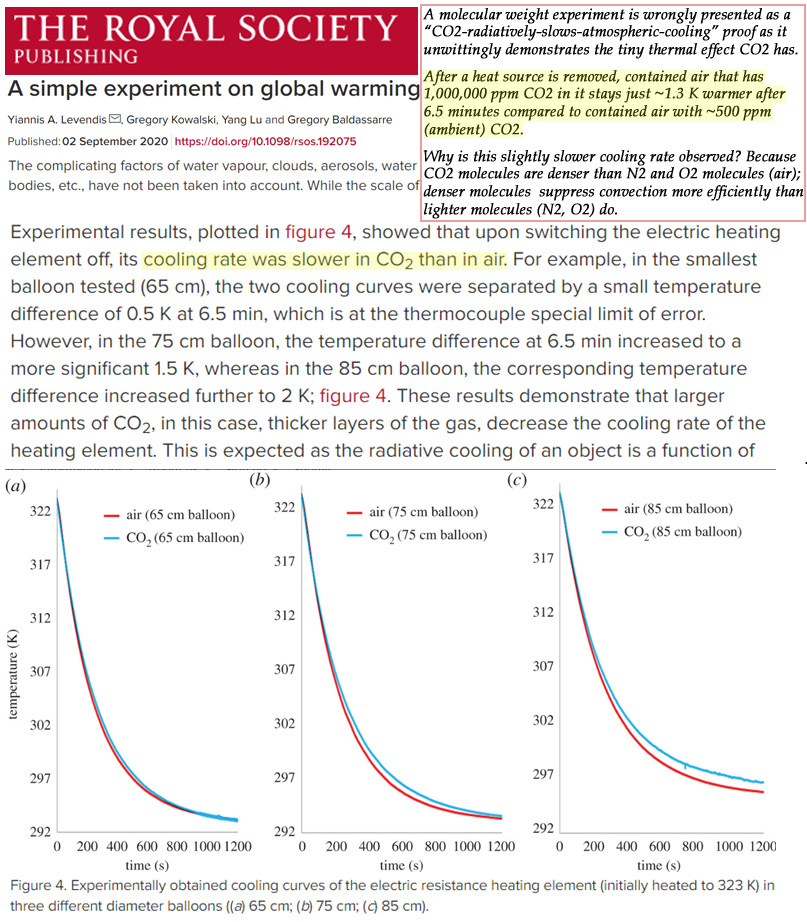
Image Source: Levendis et al. (2020)
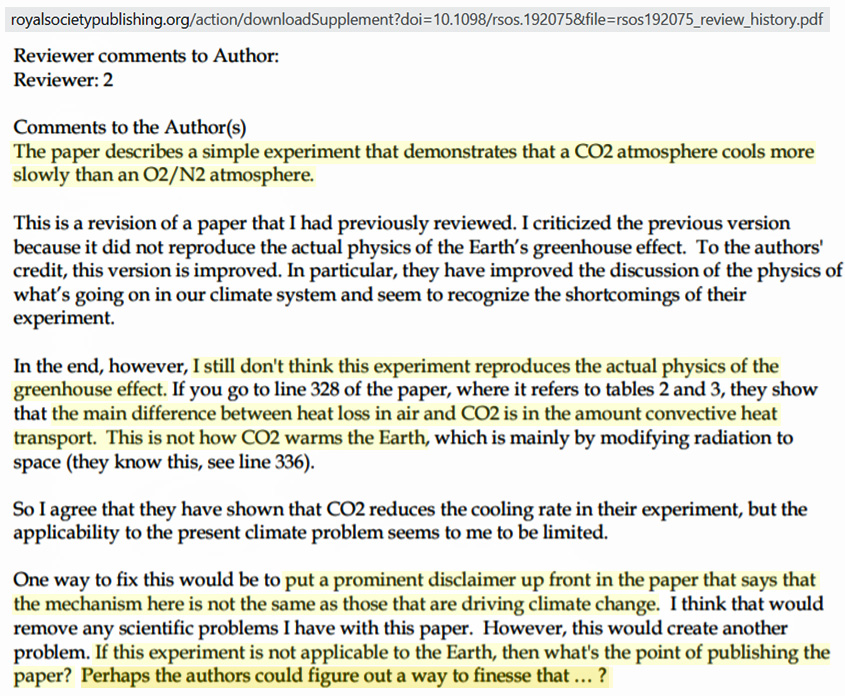
Image Source: Review history for Levendis et al., 2020
Reviewer #2 is correct. These lab experiments claiming to show CO2’s special role in the greenhouse effect only demonstrate denser molecules like CO2 (44 u) slow heat loss better than (29 u) lighter (N2 and O2, air) molecules, just as a heavier coat slows cooling better than a lighter coat.
In fact, Argon (Ar) has a much larger representation in the atmosphere (~9,000 ppm, or 0.9%) than CO2 does, but it is not considered a greenhouse gas that plays a role in the greenhouse effect. Yet as Wagoner et al. (2010) point out, Ar can be shown to reduce cooling (or “cause” warming) at an identical rate and magnitude as CO2 molecules do in experiments like these because both Ar and CO2 have similar molecular densities (40 vs. 44 u) whereas N2 and O2 (29 u) do not.

Image Source: Wagoner et al., 2010
Wagoner and colleagues clearly affirm their support for the anthropogenic global warming (AGW) position in their paper, but they lamentably admit that a demonstration of CO2’s special and significant greenhouse effect role “is difficult to demonstrate convincingly.” This is because the magnitude of the CO2’s radiative effect is already “more than an order of magnitude smaller” than observed in experiments like these that merely affirm the larger convective cooling reduction for denser vs. lighter molecules.
A real-world demonstration of CO2’s “specialness” as a greenhouse gas has yet to be observed.





I do not understand how you could have convection inside the balloon. Conduction not convection, seems more plausible.
Heated molecules inside strike the inside of the balloon, which warms, then transfers that heat to air molecules hitting the outside of the balloon. Now the molecules that struck from the inside are slower (cooler), and the remaining hot ones inside impact the balloon more frequently, thereby cooling them, as well, et., etc. The heat outside is carried away and diluted in the (presumably) much larger reservoir of the atmosphere.
It’s pretty much all convection.
OK. I need to make some “corrections.”
1. Technically it is conduction, because large amounts of gas aren’t moving around, which is what is normally referred to as convection.
2. This kind of conduction is theoretically mediated by molecular motion (which is “convection” when large parcels of them move around together).
https://www.nuclear-power.net/nuclear-engineering/heat-transfer/thermal-conduction/thermal-conductivity/thermal-conductivity-of-fluids-gases-and-liquids/
Co2 being the larger molecule, “conducts” heat more slowly than the lighter air, so it would be expected that the balloon would cool more slowly when filled with CO2 than with air. As the reviewer mentioned, it’s a known phenomenon, and doesn’t involve radiation.
I have often wondered why there aren’t more controlled experiments to test out the effect of rising CO2 levels. Does anyone want to wager that if they had enriched air to have 800 parts per million of CO2 instead of using 100% co2 that in there would be no difference in cooling?
I would take that wager. I’ve witnessed it myself that the temperature doesn’t change at all when the CO2 in a room rises from 500 ppm to 2000 to 3000 ppm.
Congratulations these scientists have confirmed my long held assertion that if CO2 did any warming, or reduced heating of the atmosphere, it would be so small as to be lost in the natural chaotic variation of the weather/climate.
At any one time the solar derived temperature difference that sets-up our weather, from tropics to poles is as much as 30°C. Atmospheric CO2 at 400ppm can not ever majorly impact that with any significant warming (or slowing of cooling) that could affect the climate.
All this nonsense about human’s controlling the climate through atmospheric CO2 venting, is just that — nonsense! Europe, especially Germany has wasted €billions on this fraud, it is about time it stopped!
Nature, not man, controls the atmospheric CO2 levels.
Nature, not man, controls the climate!
The greenhouse effect has to do with heat loss by radiation.
This experiment has nothing to do with radiation. Insteat it’s measuring some combination of conduction/convection.
I explain further below regarding another experiment making the same claims …
w.
http://wattsupwiththat.com/2013/02/06/the-r-w-wood-experiment/
Yet another crass idiotic example where temperature energy, energy flow, heat etc. are illogically mangled together. This does not represent this planet’s atmosphere. Just the usual utter nonsense from Willis!
Thomason, your comment doesn’t provide one scrap of information about just what you think might be incorrect. Waving your hands and screaming WILLIS IS WRONG goes nowhere … come back when you are willing to quote what I said that you think is wrong, and tell us why, and we can discuss it.
w.
Willis,
the atmosphere has all to do with convection, conduction radiation and in case of Earth with latent heat.
Your claim that the “greenhouse effect” is radiation only, dismisses three equally or even more important processes.
If you wanted to explore the truth, try understanding the post from LOL below
https://notrickszone.com/2020/12/03/a-new-experiment-finds-only-a-1-3c-temperature-differential-for-contained-air-with-0-05-versus-100-co2/#comment-1310450
Sorry mate but you got that one wrong!
Willis, you can not separate radiative from convective forces in the atmosphere. It is a huge intellectual mistake to try and model radiative and convective forces separately where there is mass involved. As we all know, the greenhouse effect is poorly named. There is a reason why there is no experimental evidence that shows increased CO2 concentration in earth’s atmosphere causes surface temp to increase.
Thanks, Nelson. If you think that I have “separated radiative from convective forces in the atmosphere”, please quote exactly what I said. I don’t know what you are referring to.
w.
“The greenhouse effect has to do with heat loss by radiation.”
So you did not mean to say that?
This is a quote from your post before.
The “Greenhouse effect” has to exist in a conductive, convective and radiative environment.
Taking any of the important parts out of its context will yield wrong results.
This is what Nelson tried to tell you!
I think Willis beat me to it (only because I was going to put this in last evening but ended up working too late and just wanted to turn in), but I also don’t see the relevance of this experiment to the so-called “greenhouse effect” – and I’m not sure why the authors are making such a claim.
The experiment they describe basically involves putting heated gas into a closed container, and then observing the cooling rate. Since the container is just a single chamber, the motion of the gas molecules will be uniform throughout, and will randomize the energy content (and thus the temperature) inside the container. As long as the temperature inside the container is larger than the temperature outside the container, thermal energy will be transferred (i.e., lost) via conduction at the surface of the container.
I can’t think of a reason off the top of my head why the pure-carbon-dioxide container would cool more slowly than the container filled with nitrogen and oxygen (i.e., basic atmospheric air). The specific heat of carbon dioxide is actually less than that of nitrogen and oxygen – so for the same temperature increase (i.e., if you heat each mixture to the same temperature), there is actually more energy in the nitrogen/oxygen mix than in the carbon dioxide. But there are A LOT of other things going on that would require detailed analysis – such as the amount of material in the container for the same pressure or something (carbon dioxide is a much heavier molecule than either nitrogen or oxygen for example).
Two or three years back, when Pierre had a couple of regular stalker-trolls, one of them made some moronic comment comparing “the greenhouse effect” to double-glazed windows – and I had to explain to him how double-glazed windows work, and why the region between the two panes (hence the name “double-glazed”) is filled with a noble gas like argon or neon. This experiment seems to be closer to that type of situation, but it’s basically a single-glazed window – the gases cannot cross the boundary, but thermal energy can (by conduction).
The “greenhouse effect” postulate is based entirely on radiative transfer. The sun emits radiation as basically a blackbody spectrum with a peak in the visible-yellow region. When this reaches the land surface of the Earth, the visible light is absorbed and is stepped-down (as it were) from visible light energy to lattice vibrations (or the equivalent) in the solids of the surface. These lattice vibrations are at low frequencies (i.e., longer wavelengths) than the incoming visible light – and are basically in the infrared. Thus, the surface emits infrared radiation – which can either escape to space, or be absorbed by any molecules that have characteristic resonance frequencies at the emission frequency.
The whole “greenhouse” argument is that carbon dioxide is a critical such absorption molecule – and that more carbon dioxide means more absorption of this surface-emitted infrared radiation. The other piece of the argument is that the surface-emitted radiation is directional (outward bound), but when a carbon dioxide molecule absorbs an infrared photon and enters an excited state, when it decays from that excited state and re-emits an infrared photon, that emission will be directionally-random with respect to an imaginary sphere that surrounds the molecule; thus, some of the re-emitted infrared photons will head back down to the surface, where they are absorbed by the surface as lattice vibrations, manifesting themselves as more heat. The net of the argument is that more carbon dioxide interferes with the emission of infrared radiation from the surface to space – sending more of it back to the surface.
The term “greenhouse effect” is actually a poor analogy – which is of course misinterpreted by the uninformed (as the old stalker-trolls used to regularly do). A real greenhouse works by blocking convection and advection – in other words, it prevents warm air from moving away and being replaced by cooler air. In other words, it’s why you keep your doors and windows closed during the winter months.
The real experiment would be to do something like set up two (identical) greenhouses next to each other – and to let one have plain old atmospheric air and the other to have an “enhanced” concentration of carbon dioxide within. If the “greenhouse effect” is materially real, then the second greenhouse should warm up to a higher temperature than the first one.
BTW, this experiment is apparently run regularly by commercial greenhouse operations. They pump more carbon dioxide into the greenhouse (something like 3x atmospheric concentration if I recall correctly, but anyone can look this up if they are interested). They don’t do this to increase the temperature – they do this to enhance plant growth. (And seriously, if you could increase the heat markedly by just adding carbon dioxide to the air, it would be a marvelous source of energy, wouldn’t it? Put in enough carbon dioxide, and I could use the heat to make steel. 🙂 )
Sorry that go so long. Science is difficult. 🙂
Thanks, you made some very good points.
Thanks!
I actually looked at Figure 4 a little more closely, and this gets weirder.
The behavior of the 65cm balloon looks like about what I’d expect – both cool at essentially the same rate and reach thermal equilibrium with the outside at the same time.
But the behavior changes when the only change to the experiment is using larger balloons (75cm and 85cm)?! This really doesn’t make any sense – the size of the balloon shouldn’t be material. Those two curves don’t go to completion for whatever that’s worth – they both show one warmer than the other, but that can’t be correct unless they left off the rest of the graph where they both reach the same temperature (as mandated by basic thermodynamics).
(N.b. – As an aside, remind me again why “peer-reviewed” material is someone Absolute Truth (TM) – when something like this gets by “peer review”…)
“the size of the balloon shouldn’t be material.”
That depends on what’s the rate limiting step. Is heat carried away from the surface faster on the outside than it can be transferred to the surface on the inside.
The original works of the American Frank Very showed silmilar results.
ftp://ftp.library.noaa.gov/noaa_documents.lib/NOAA_historic_documents/WB/Bulletin/Bulletin_G.pdf
Ironic that NASA hold this document.
That is a nice find. Thanks, ACO.
Didn’t they just compared the thermal conductivities of different gases ?
Indeed, at same pressure and temperature, CO2 thermal conductivity is lower than air’s one :
https://www.engineeringtoolbox.com/air-properties-viscosity-conductivity-heat-capacity-d_1509.html
https://www.engineeringtoolbox.com/carbon-dioxide-thermal-conductivity-temperature-pressure-d_2019.html
Thus the air balloon should cool faster than the CO2 balloon.
But how such air+CO2 conductivity modification induced by say, doubling CO2 concentration (from 400 to 800 ppm) could have any measurable effect on the cooling ability of the atmosphere with respect to other involved processes (evaporation, cloudiness, turbulent convection, radiation, …) ?
I actually don’t think that the thermal conductivity of the gases is material. The thermal conductivity that matters is the thermal conductivity of the walls of the container – so that if you use the same container, the thermal conductivity of the walls should be the same and the heat transfer through them will be at the same rate.
Rate behavior should be based on the specific heat – as each gas will take a different amount of energy to heat it to the same temperature. But I don’t even see how that is material to this particular experiment (off the top of my head) – since each is acting as a thermal reservoir of the Carnot type.
It’s like an RC circuit in electrical engineering – the thermal conductivity (really, its inverse) is R, and the specific heat (again, really its inverse) is C. Neither one determines the endpoint – just the rate at which you get there. And the only material thermal conductivity here is the thermal conductivity of the walls of the vessel.
You know… what they might have actually measured is the difference in the thermal conductivity between different balloons! Two different balloons won’t be identical, but will have been subject to manufacturing variations. The way to do this experiment would be to use the same balloon sequentially for each gas – and to repeat the cycles to make sure that the inflating and deflating of the balloon isn’t itself changing the thermal conductivity of the balloon wall material (which is possible – rubber materials do very strange things).
That’s actually the best explanation that I can come up with for Figure 4!
Of course, that experiment completely negates the actual effect that CO2 has in cooling the atmosphere.
CO2, being a polyatomic molecule, has higher specific heat capacity than the homonuclear diatomics (so of course it’s going to cool off more slowly via conduction, it can store more heat than the homonuclear diatomics). In the atmosphere, this means a higher CO2 concentration will absorb more energy at the surface via conduction (as compared to the homonuclear diatomics) and thus convect more energy to the upper atmosphere.
That’s where the balloon experiment cuts it off. But there’s another effect… that of radiative emission to space of that convected energy.
An atmosphere with a higher CO2 concentration will more effectively radiatively emit, increasing photon flux and thus increasing photon flux out to space. The balloon material absorbs the photons and thus traps that energy, to be reabsorbed by the CO2 in the balloon, slowing cooling.
The extinction depth for 14.98352 um radiation in the troposphere is only ~10.4 m (and doubling concentration of CO2 would reduce that to ~9.7 m), so any energy that would be thermalized at double CO2 concentration is already being thermalized in the troposphere… the net effect in any case is an increase in CAPE (Convective Available Potential Energy) which increases convection, which carries energy entrained in that higher specific heat capacity of CO2 (as compared to the homonuclear diatomics) and the latent heat capacity of water to the upper atmosphere, where it radiatively emits, with the majority of that energy going out to space due to the fact that mean free path length for that radiation decreases exponentially with decreasing altitude and increases exponentially with increasing altitude… so any downwelling radiation is absorbed and re-emitted upwelling in short order.
Now, given that the lapse rate is ‘anchored’ at TOA (Top of Atmosphere, that altitude at which the atmosphere effectively becomes transparent to any given wavelength of radiation), and given that polyatomic molecules shift the lapse rate vertically (reference the difference between dry and humid adiabatic lapse rate, for instance), that means that an increasing atmospheric concentration of polyatomic molecules increases thermodynamic coupling between heat source (surface) and heat sink (space) (a higher concentration of polyatomic molecules will shift the lapse rate more vertically). IOW, they transit more energy, reducing the temperature differential between source and sink. Given that the sink in this case is space and it is essentially an infinite heat sink, it’s the temperature of the planet that will change due to that more-vertical lapse rate, not the temperature of space.
So polyatomic molecules act as atmospheric coolants, not ‘greenhouse gases’… with water acting as a literal refrigerant (in the “refrigeration cycle” sense… it absorbs energy at the heat source (surface), undergoes phase change (liquid to vapor), is transported, emits that energy and undergoes phase change (vapor to liquid) and sometimes emits more energy and undergoes another phase change (liquid to solid), is transported and repeats the process… just as occurs with a refrigerant in the refrigeration cycle).
The homonuclear diatomics are the ‘greenhouse gases’… they can absorb energy via conduction with the surface, they can convect to the upper atmosphere, but once there they cannot as effectively radiatively emit that energy because they don’t have a net magnetic dipole and require a collision to perturb the molecule which perturbs the magnetic dipole, allowing the molecule to absorb or emit. But in the upper atmosphere, air density is low and collisions happen far less often.
Thus, in an atmosphere of only homonuclear diatomics, the upper atmosphere would warm up because it could not as effectively radiatively emit energy it’d picked up from the surface via conduction. That would mean the lower atmosphere would have less buoyancy, which would hinder convection and would thus hinder convective cooling of the surface (convection and evaporation carry away ~76.2% of surface energy). Thus the surface would have to warm because the surface would need to shed energy via radiative emission alone in the amount that it used to shed via radiative emission and convection / evaporation.
That hindrance of convection in a solely homonuclear diatomic atmosphere would be the closest to an actual “greenhouse effect” (a restriction of convection), which the climastrologists attempt to conflate with tropospheric thermalization (which only increases CAPE and thus increases convection).
CO2 has actually a lower Cp than Nitrogen.
The conductivity is roughly equal (Nitrogen a bit higher).
The experiment has to many error sources and good suggestions have been made here to improve it.
Drawing any conclusion out of the current results seems premature and a conclusion towards the “greenhouse effect” not possible, without confirmation that the model actually reflects true atmospheric conditions.
It seem it does not as you point out in you post.
Very interesting to say that the lapse rate is anchored at TOA.
As I believe you are right with this one, there is one interesting conclusion to be drawn.
Since the dry lapse rate is higher than the wet adiabatic lapse rate, theoretical temperatures at the surface for the dry lapse are actually higher than for a water saturated atmosphere.
I believe this is true and is indicative of an increased heat transfer rate in presence of water through the atmosphere.
This was a heat capacity experiment. And they neglected to show heating CO2 balloon taking longer to heat!
This is scientific fraud.
This exercise ball experiment appears to show that there is indeed a CO2 slowing-of-cooling effect in a simulated atmosphere less than half a meter thick.
It is done very competently and it’s agreement of measurements with theory is impressive.
However it’s interpretation is not straightforward. For instance as Clive Best pointed out CO2 IR absorption saturates in effect after only 25m:
The absorption length for the existing concentration of CO2 is around 25 meters i.e. the distance to reduce the intensity by 1/e.
https://ptolemy2.wordpress.com/2020/07/05/clive-best-its-a-circular-argument-if-we-assume-co2-warms-the-earth-we-find-that-co2-warms-the-earth/
Now this Royal Society experiment by Levendis et al. operates over gas distances of just a few cm, so it is different from the atmosphere in that it is at a scale where the CO2 IR absorption effects are well below saturation. This will influence interpretation of the study and its significance to the earth’s surface and atmosphere.
However even at the small scale of the rubber exercise ball, it looks like there might be a signature of incipient saturation. Take the results of the difference between cooling curves at different ball diameters. Ball diameters of 65, 75 and 85 cm were used. Here are the associated temperature differences that they found:
For example, in the smallest balloon tested (65 cm), the two cooling curves were separated by a small temperature difference of 0.5 K at 6.5 min, which is at the thermocouple special limit of error. However, in the 75 cm balloon, the temperature difference at 6.5 min increased to a more significant 1.5 K, whereas in the 85 cm balloon, the corresponding temperature difference increased further to 2 K;
Now here we have a nonlinear result. Increasing diameter from 65 to 75cm increased the temperature difference by one degree. But a further similar increase of diameter by 10 more cm to 85 cm resulted in a further increase in temperature difference only half as much; only half a degree.
So one could extrapolate that further increases of the ball diameter would result in smaller and smaller increases in the temperature difference. Until at a certain diameter there would be no more significant growth in the temperature difference. In other words, saturation.
In this train of thought the expectation of a linear relationship between gas thickness and temperature difference is no doubt naive. However it does seem to point to an inevitable saturation of the demonstrated slowing-of-cooling effect, after just a few meters of distance.