Per scientists, removing a greenhouse gas – water vapor – from the atmosphere results in net additional forcing (warming). Irrigation studies also affirm adding water vapor cools the surface.
Per “mainstream” climate science, Earth’s total greenhouse effect radiative forcing from water vapor, clouds, CO2, and trace greenhouse gases (like methane) amounts to 150 W/m² (Lacis, 2018). About 50% of this 150 W/m² total (75 W/m²) greenhouse effect forcing is derived from water vapor, 25% (37 W/m²) is from clouds.

Image Source: Lacis, 2018
But the contradictory “mainstream” science says removing clouds and water vapor from the atmosphere actually has a far greater radiative impact than their influence as greenhouse warming agents.
A cloud-free day at noon results in an extra +251 W/m² of radiative forcing relative to a cloudy day at noon. Removing water vapor , a greenhouse gas, leads to a forcing increase of +131 W/m².

Image Source: van Heerwaarden et al., 2021
Together, that’s +382 W/m² of additional forcing with the absence of these greenhouse effect/greenhouse gas agents, whereas the total greenhouse effect forcing from water vapor and clouds is just +112 W/m² (75 + 37 W/m²).
So the presence of greenhouse gases (water vapor) with clouds combined have a net -270 W/m² cooling effect on Earth’s surface temperatures.
Adding water vapor to surface air leads to cooling
According to a 2018 study by Sherwood and co-authors, the last 100 years of anthropogenic water vapor emissions (mostly from irrigation) have produced a total effective radiative forcing of -0.1 to 0.05 W/m². In other words, adding more greenhouse gases in water vapor form leads to a “near-zero or small cooling effect.”
Sherwood et al. even acknowledge “large increases in anthropogenic water vapor emissions would have negligible warming effects on climate” because “greenhouse-gas warming is outweighed by increases in…humidity-induced low cloud cover.”
Image Source: Sherwood et al., 2018
New studies have continued to verify that adding the water vapor greenhouse gas to the surface air results in cooling.
For example, Kala et al. (2023) report substantial cooling results from adding this greenhouse gas.
“Our results show that irrigation could potentially reduce the mean seasonal maximum temperature during the Angry summer of 2012/2013 by −1.44°C to −2.13°C over irrigated regions.”
Zhang et al. (2023) also report expanding irrigation across regions of China has resulted in verifiable cooling effects.
“We found the space-and-time method can better represent the cooling effects of long-term irrigation expansion than the space-for-time method, despite that they derived similar cooling trends. The cooling effects were most evident in South Xinjiang, followed by North Xinjiang and Hexi Corridor, and varied in different irrigation expansion sources. Specifically, more intensive cooling occurred in new irrigated areas from unused lands (-0.69 ± 0.02 K) than that from grasslands (-0.47 ± 0.05 K) and forests (-0.28 ± 0.04 K). The cooling effects were dominated by marked daytime cooling compared to negligible nighttime warming.”
Other recent studies also affirm the conclusion that adding water vapor, Earth’s main greenhouse gas, cools the surface air.
In the very same regions of India where irrigation has been most prevalent, significant growing-season cooling has been occurring. Land surface temperatures (LST) in the Indo-Gangetic Plain have cooled by -0.8°C since 1979, and this is “associated with the irrigation expansion” in the region.
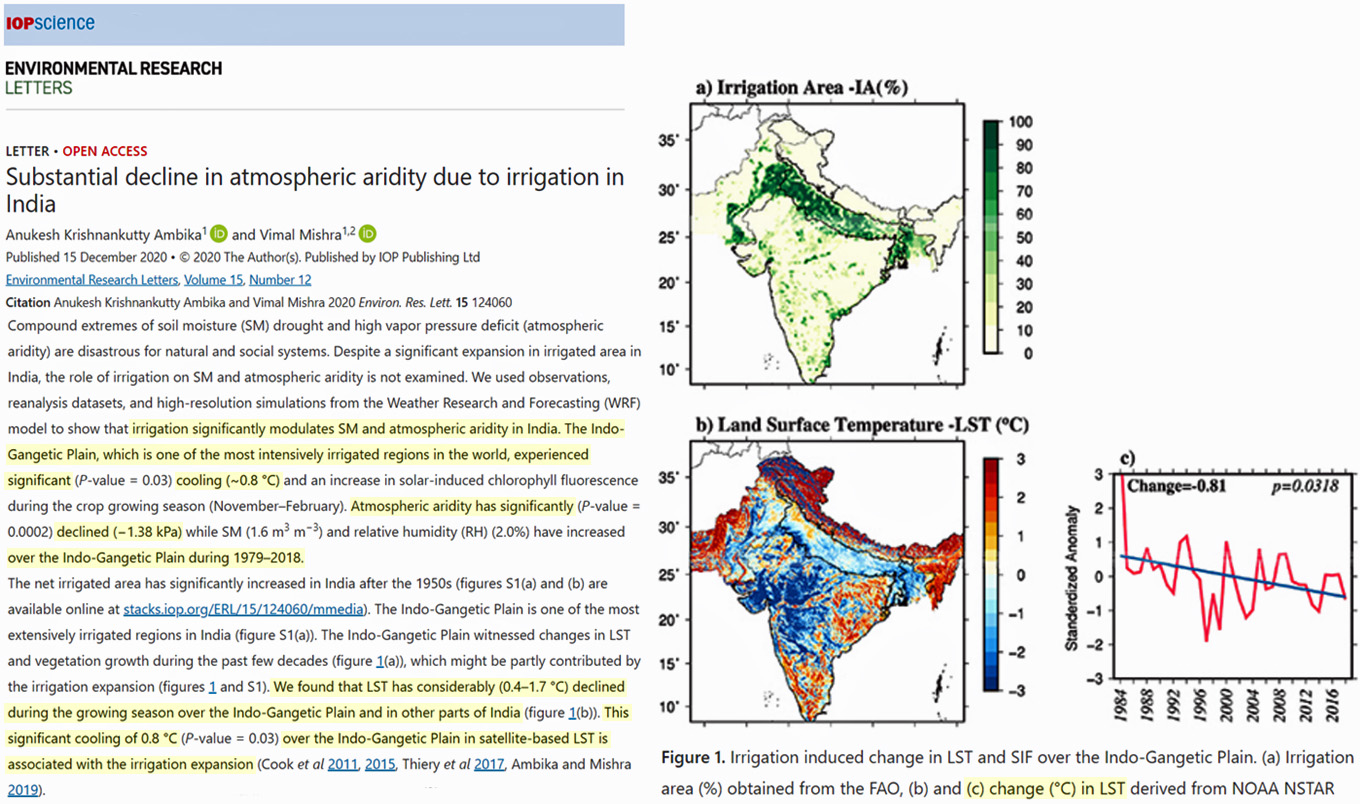
Image Source: Krishnankutty Ambika and Mishra 2021
The same phenomenon has been observed in China. Scientists (Yang et al., 2020) even refer to adding anthropogenic water vapor emissions to the atmosphere as the irrigation cooling effect (ICE), which is “closely linked to the surface energy balance.”
Irrigation “cools daytime LST by 1.15 K, and cools nighttime LST by 0.13 K, on average, across the irrigated areas of China.” During the growing season, pumping Earth’s most powerful greenhouse gas into the atmosphere results in a cooling effect of more than 6 K in irrigated (ICE) regions compared to adjacent locations not subjected to greenhouse gas cooling.

Image Source: Yang et al., 2020
Image Source: Zhang et al., 2022
These observational results would appear to contradict the modeled claims that water vapor is a positive feedback leading to enhanced warming.





There is something about green house gas effect which has troubled me from the beginning. The photons absorbed by those molecules like CO2 or methane are reemitted but the energy of those reemitted photons is lower than the absorbed ones. This is the fundamental law of Stefan-Boltzman law. The second problem is the role of water which is different from other so called green-house gases. Water evaporates and becomes a gas, when clouds form due to the temperature gradient (lapse rate) energy is released and lost in all directions : only a small portion goes back to the surface of earth. When water droplets become ice at higher altitude the same loss of energy occurs. Finally water dissipates the energy from the surface of earth (mainly the oceans) toward the space. Without water as a gas temperature would be very high during day time and very cold during the night like in a desert. The occurence of water vapor on climate is easy to understand in that way.
Exactly correct. According to Wiens law, co2 radiation at 14μm corresponds to a blackbody at ~200K. This means that the effect co2 in the atmosphere has on the surface at 14C, 287K, is cooling. A 200K body will cool a 287K body.
What´s interesting is that co2 will keep emitting at that intensity even if it´s concentration increases. Even with 100% co2 it will still act like a 200K body, which means that the more co2 the more efficient it will cool.
With water you can analyze the whole cycle and find nothing but cooling. Start with the oceans, you have 4km deep water at 3 Celsius laying on top of very thin crust on the ocean floor, which has very hot mantle underneath. Compare this to a pot on the stove, what happens if you run the boiler plate without water? It gets red hot, but if you fill it with water it cools down massively. It´s safe to assume that the same applies to the oceans, if you´d remove the oceans the planet would become very hot. This is most probably the reason for Venus being so much hotter than Earth, it has no water cooling the surface. Earth would be much hotter without water.
Then we can look at evaporation. Evaporation happens as water molecules absorb heat and breaks the bonds of the liquid. Evaporation cools both the water surface and the air as relative humidity rises. This principle is used for swamp coolers, we know this as a fact. The water molecules then carries the heat away from the surface internally, and this is a process of thermodynamic work, displacement of mass. Work is a cooling process when performed BY a system. Heat is transformed into work, and this will cool. Then, as water condense at altitudes of low temperature, the heat is dumped into the cold atmosphere. This is how you´d construct a cooler, move the heat from a hot place to a cold place and dump it there. As condensation happens, clouds blocks the external heat source(sun), this will cool the system even more. And when rain falls the cold droplets will cool the surface even more as the land.
From start to finish the water cycle is a cooling process. The idea of water as a warming agent is utterly stupid and it has no basis in physics at all. Nobody has ever shown water to have a warming net effect on any system in experiments. It´s completely made up.
“This means that the effect co2 in the atmosphere has on the surface at 14C, 287K, is cooling. A 200K body will cool a 287K body.”
Good show! You are right and I am pleased to hear it, but all I have read, including most recently an experiment by Dr. W.J. Witteman in 2020, places CO2’s major peak displacement wavelength at 15μm, with an interactive temperature t 193.13K or minus 80 Celsius degrees. The interactive range there is from 13.26 to 17.07 microns. Witteman found the next strongest interaction is 9.6 μm, with a temperature of 301.77K. This presumably accompanies other reactive gases at the 8 to 12 μm wavelength range through the “window” direct to space. Dr. Salby states that with them it warms the ozone layer. Witteman found lesser reactions peaking at 4.3, 2.7 and 2.0 μm, with temperatures from 400 to 1175 Celsius degrees but in trivial amounts of about one photon per 158,750 molecules. (Witteman, W.J., “The absorption of thermal emitted radiation by CO2,” April 3, 2020, https://principia-scientific.org/the-absorption-of-thermal-emitted-infrared-radiation-by-co2/).
The cooling effect of evaporation and the warming effect of condensation is due to the molecule’s absorption and release of latent heat in the reactions. Of course, solar heat absorbed for evaporation is unavailable as radiant heating of the planet, and meteorological forces move the heat to cooler altitudes and altitudes where it is a benefit.
Oh well, always a typo. With me they seem inevitable. … Should be “cooler altitudes and latitudes.”
So I’ll be warmer outdoors on a sunny day than I will be on a cloudy day? What complete nonsense!
[…] From NoTricksZone […]
California’s Central Valley is probably an example of how you can remove water vapor and increase temperatures. The Valley had 4 million acres of wetlands in the 1850’s and it declined to 400,000 acres by 2000 and may be half that now. The California water project channeled water and mountain runoff into aqueducts that distributed it for irrigation. A little more than a decade ago, a third of this water was diverted to environmental discharge to save an endangered darter species. The big coastal metropolises also get a large fraction of the central valley project waters. Farmers, who see the greatest cuts in allocations during dry years, resorted to pumping groundwater to make up for the lost water. However, ground water was recently restricted. As a result, the central valley saw record heat at the end of last summer. The state blames climate change but the anthropogenic part of this may be the water diversions to the coasts and the ocean.
This reminds me of Dr. Jim Steele’s comment that ultimately climate is local.
Looks like an appropriate time to revisit these.
Water vapor feedback is negative.
https://m.youtube.com/watch?v=Y2K1uHvfaek
A warmer world is a wetter world (t = 26:45-27:32)
https://m.youtube.com/watch?v=z_OjGxrlloE&t=212s&pp=2AHUAZACAQ%3D%3D
Did this extra CO2 we’ve been creating save us from another ice age?
I’ve been saying the same for years… all polyatomics are net atmospheric radiative coolants (with water acting as a literal refrigerant (in the strict ‘refrigeration cycle’ sense) below the tropopause and with other polyatomics contributing comparatively less cooling below the tropopause but with CO2 being the prevalent atmospheric radiative coolant above the tropopause); and all monoatomics (which have no vibrational mode quantum states and thus cannot emit IR to space) and homonuclear diatomics (which have a net-zero magnetic dipole and thus cannot emit IR to space unless that net-zero magnetic dipole is perturbed via molecular collision) act as dilutants to the polyatomics which can emit to space.
The refrigeration cycle (Earth) [A/C system]:
A liquid evaporates at the heat source (the surface) [in the evaporator], it is transported (convected) [via an A/C compressor], it gives up its energy to the heat sink and undergoes phase change (emits radiation in the upper atmosphere, the majority of which is upwelling owing to the mean free path length / altitude / air density relation) [in the condenser], it is transported (falls as rain or snow) [via that A/C compressor], and the cycle repeats.
In at atmosphere consisting solely of monoatomics, they could pick up energy via conduction by contacting the surface (just as the polyatomics do), they could convect (just as the polyatomics do), but once in the upper atmosphere, they could not radiatively emit that energy to space. The upper atmosphere would thus warm (and due to gravitational auto-compression, so too would the surface necessarily warm), lending less Convective Available Potential Energy to parcels of air attempting to convect, thus convection would be hindered. And that’s how an actual greenhouse works… by hindering convection. The surface radiant exitance would thus have to pick up that ~76.2% of surface energy which is currently removed via convection and evaporation… and a higher surface radiant exitance implies a higher surface temperature.
For an atmosphere consisting of monoatomics and homonuclear diatomics, there would be some collisional perturbation of the homonuclear diatomics’ net-zero magnetic dipole and thus some emission in the atmosphere, but not nearly so much as from polyatomics.
Polyatomic molecules (CO2 , H2O) reduce the adiabatic lapse rate (ALR), not increase it (dry ALR: ~9.81 K km -1 ; humid ALR: ~3.5 to ~6.5 K km – 1) by dint of their higher molar heat capacity and/or latent heat capacity convectively transiting more energy (as compared to the monoatomics and homonuclear diatomics), thus attempting to reduce temperature differential with altitude, while at the same time radiatively cooling the upper atmosphere faster than they can convectively warm it… they increase thermodynamic coupling between heat source and sink… they are coolants.
The dry adiabatic lapse rate is primarily due to the monoatomics (Ar) and homonuclear diatomics (N2, O2).
S. Ackerman et. al, Maria Z. Hackuba et. al (NASA JPL), Clough & Iacono and several others corroborate the above in peer-reviewed studies.