Since the early 1990s the conventional assumption, aligned with modeling, has been that a molecule of human CO2 emission stays in the atmosphere – its residence time – for centuries. This fits the anthropogenic global warming (AGW) narrative. But empirical evidence contradicts these model-based assumptions. Residence time is closer to 5-10 years.
In Table 1 of a new study, Stallinga (2023) compiled a list of 36 published estimates of CO2 residence time spanning the decades 1957-1992. All of these scientists determined CO2’s atmospheric residence time is about 5 to 10 years or less.
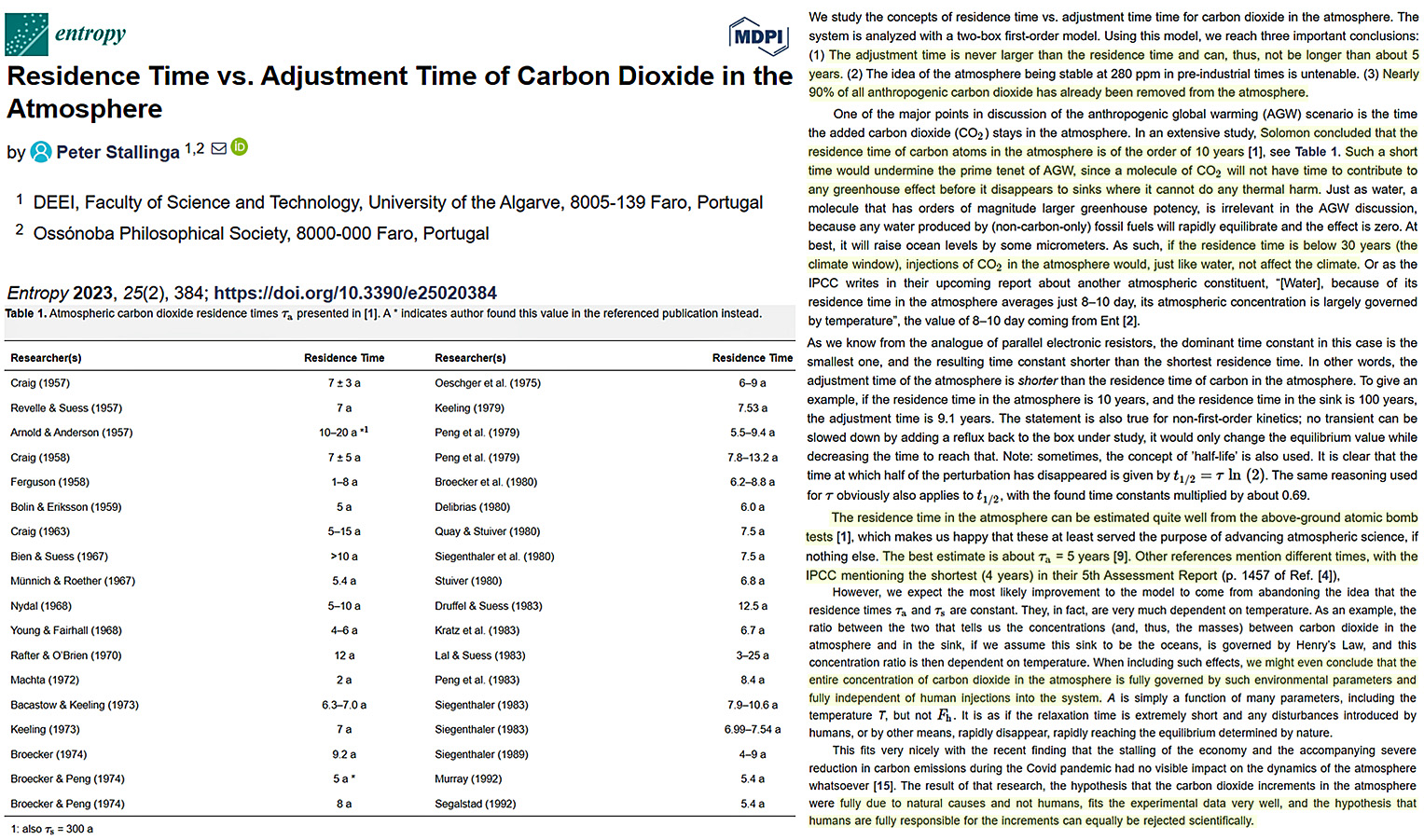
Image Source: Stallinga, 2023
Of course, these were the pre-IPCC decades in climate research, when “the science” was pursued independent of government interference. For example, it was still acceptable in the 1950s to early 1990s for scientists to publish actual ice core measurements showing the atmospheric CO2 content ranged up to 700 ppm, even 2,450 ppm, in the ice sheets and glaciers examined throughout the last 10,000 years (Jaworowski et al., 1992).
An actual residence time that is 20 to 40 times shorter in duration than what an AGW modeled thought experiments allow undermines the dangerous greenhouse gas accumulation talking points, as “if the residence time is below 30 years, injections of CO2 in the atmosphere would, just as water, not affect the climate” (Stallinga, 2023).
In addition to compiling an exhaustive list of past estimates supporting a 5-10 year residence time, Dr. Stallinga cites the evidence from atomic bomb tests, the lack of any atmospheric CO2 effect from the pandemic lockdowns and associated sharp drop in emissions, and the lead-lag relationship CO2 emission has with temperature as evidence supporting the once commonly-accepted conclusion that CO2 residence is closer to 5 years, not centuries.
And if residence time is only 5 years, nearly “90% of all anthropogenic carbon dioxide has already been removed from the atmosphere.”
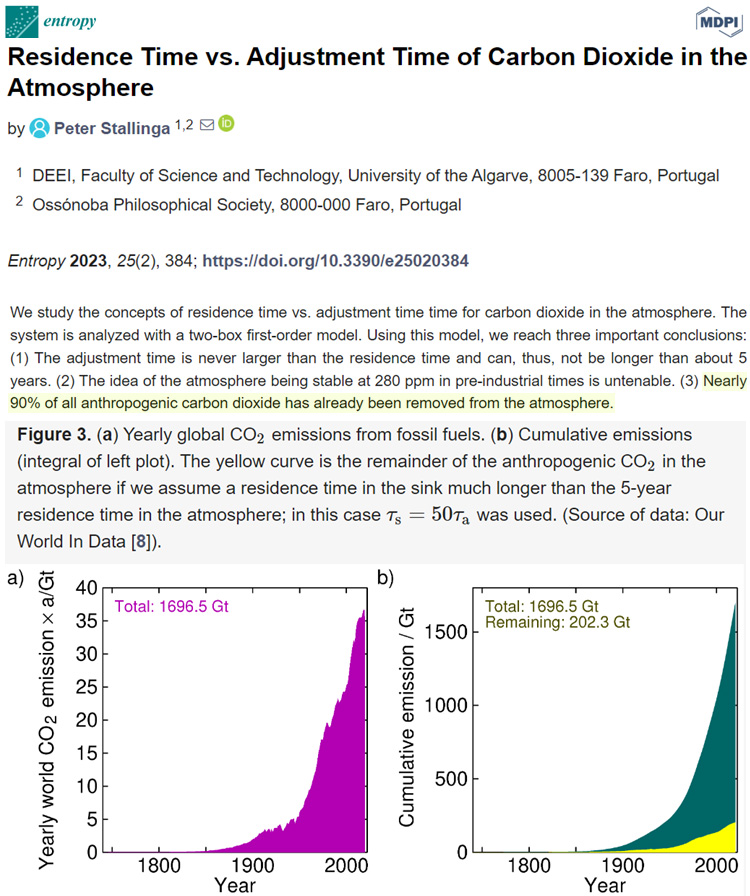
Image Source: Stallinga, 2023
Stallinga’s conclusions are very similar to that of Dr. Chauncey Starr (1993), a nuclear physicist who emphasized that a centuries-long residence time claims are derived from “global carbon cycle models which are adjusted to fit the assumption that anthropogenic emissions are primarily the cause of the observed rise in atmospheric CO2.”
Starr provided empirical support for the conclusion residence time is 4-5 years. For example, the high altitude 1964 nuclear bomb testing revealed how quickly (3-11 years) the atmosphere cycled through these perturbations. The constancy of the amplitude of seasonal cycle of CO2 and the magnitude of the swing in annual concentrations are both consistent with photosynthesis as the driver of CO2 variability, further affirming a residence time of 5 years or less.
Also, similar to Dr. Stallinga’s analysis, Starr reported “only about 15% of the fossil-fuel emissions remain in the atmosphere.”






https://www.mdpi.com/1099-4300/25/2/384
This is a revolutionary paper. It undermines almost all of the IPCC assumptions on atmospheric CO2 history, stability and time parameters of residence. It supports anomalous and contradictory CO2 data that has been discarded as erroneous.
It seems straightforward. I’d love to see it being challenged.
Let’s see if it gets any traction.
The question they leave unasked/unanswered is where the increase in natural emissions of CO2 occurs. I suggest we consider the loss of snow and ice cover at the end of the LIA exposed more sea water to devastating and decaying plant matter in high altitude areas (the glaciers and ice and snow overrode plant matter, did not bulldoze it out of the way). To test this one might look to the sea water CO2 content under Antarctic ice shelves with low current flow to see if there is a CO2 buildup there. Same with under land based glaciers.
Bill Gates says CO2’s lifetime is thousands of years.
Meanwhile, the CO2 fluxes between 280 and 980 ppm within hours at the surface above meadows, and between 300 and 600 ppm over tree cover.
Douglas,
It is the same error that many before Stallinga made: from Segalstad (1997) to the late Salby, Berry and Harde (2023)…
Stallinga indeed mentions the difference between residence time and relaxation time, but doesn’t take the difference into account in his further reasoning.
The residence time is about how much CO2 is passing a CO2 mass in a container and can be expressed as:
RT = mass/input = mass/throughput = mass/output
as long as input = throughput = output in equilibrium.
In most cases mass/output is used, but strictly mathematically, mass/throughput is right. RT is independent of the direction of the fluxes. Even if inputs changed to outputs half the year and reverse (which is the case for the seasonal CO2 fluxes) the sum of all absolute CO2 fluxes counts together for the residence time.
For the atmosphere that makes:
884 PgC / 210 PgC/year = 3.8 years
That is about how much CO2 is passing by in the atmosphere and doesn’t change the total amount of CO2 in the atmosphere with one gram.
It does change the possibility of an individual CO2 molecule to get EXchanged with a CO2 molecule from another reservoir. That is what Stallinga in fact calculated.
The relaxation time is from a complete different order and for a linear process in dynamic equilibrium is expressed as:
Tau = disturbance / effect.
For a linear process (which the removal of CO2 out of the atmosphere is) that is the time to get 1/e or about 37% of the initial disturbance. That gives:
Tau = 120 ppmv above equilibrium / 2.25 ppmv net sink/year = 53 years
Or a Tau of around 50 years or a half life time of around 35 years.
Two totally different definitions and totally different results…
“RT is independent of the direction of the fluxes.”
Residence time is the average time CO2 molecules reside in the reservoir. So, it’s defined per reservoir and not bi-directional. If two interacting reservoirs at equilibrium do not have the same mass, they have different residence times. As Stallinga states: MassAtm/MassSink = RTa/RTs.
“The relaxation time is from a complete different order and for a linear process in dynamic equilibrium is expressed as: Tau = disturbance / effect.”, “Tau = 120 ppmv above equilibrium / 2.25 ppmv net sink/year = 53 years”
It seems that you believe in some magical equilibrium, with an invented “net sink”. The IPCC defines Adjustment time as: the time for atmospheric CO2 to re-equilibrate following a perturbation. Putting extra CO2 in the atmosphere (due to e.g. fossil fuels) is such a perturbation of disturbance. The extra CO2 in the atmosphere changes the mass of the reservoir and therefor the flow to the sink (based on the equation F = M/RT). Stallinga gives a clear explanation of this equilibration process, and shows that the adjustment (or relaxation) time can never be longer than the shortest of the two RT’s.
See e.g. http://postimg.cc/K4rTMm0P
Dear Frans,
The residence time of CO2 in the atmosphere is certainly bidirectional:
In the case of the atmosphere, three reservoirs are involved: oceans, atmosphere and vegetation.
In spring/summer:
An estimated 50 PgC moves from the warming ocean surface (+14 K in the NH land + sea) through the atmosphere while 60 PgC is absorbed by growing new leaves in vegetation and further photosynthesis.
In fall/winter the opposite happens (thus reverting the CO2 fluxes through the atmosphere).
Net result: increasing drop of CO2 in the atmosphere in the NH spring/summer with increasing temperatures and reverse in fall/winter causing a seasonal amplitude of about 10 PgC or 5 ppmv.
Completely temperature dependent, practically independent of the total CO2 amount in the atmosphere.
Diurnal: estimated +/- 60 PgC day/night totally temperature dependent and independent of total CO2 in the atmosphere.
Equator-Poles: estimated 40 PgC/year, returns some 1,000 years later via the deep oceans (but near the same mass of “old” CO2 returns in the same year). Temperature difference dependent, hardly dependent of total CO2 in the atmosphere.
Total fluxes: 50 + 60 + 60 + 40 = 210 PgC.
RT = 884/210 = 3.8 years.
Net removal rate of any CO2 out of the atmosphere: near zero.
The residence time in the case of the natural fluxes is near entirely temperature dependent, bidirectional for the diurnal and seasonal fluxes and a simple cycle for the equator-poles flux.
In al cases hardly dependent of the total pressure in the atmosphere or the extra CO2 pressure above the all time equilibrium.
There is an all time dynamic equilibrium between ocean surface and atmosphere of about 16 ppmv/K over the past 800,000 years (glacial to interglacial and reverse, with a “speed” of 0.02 ppmv/year). The current 120 ppmv in 170 years from 200 ppmv human emissions has nothing to do with natural fluxes or exchanges between the different reservoirs…
Per Henry’s law the current SST gives an equilibrium with the atmosphere of around 295 ppmv. That can be calculated with the formula of Takahashi starting from 280 ppmv during the LIA and an increase of 0.8 K in SST:
∂ln pCO2/∂T=0.0423/K or about 4%/K. 15 ppmv CO2 increase since the LIA, that is all.
The relaxation time is the effect of the 120 ppmv extra pressure in the atmosphere above the all-time equilibrium which pushes 2.25 ppmv/year into oceans (and vegetation). The latter is the observed difference between human emissions and increase in the atmosphere, thus the net sink rate in nature. That gives a relaxation time of around 50 years or a half life time of around 35 years. That is the only part in all equations and fluxes that is pressure (difference) dependent and not temperature dependent.
Last but not least: One may reverse the formula of the RT if and only if all fluxes are unidirectional from input to output, thus never for bidirectional fluxes.
Or how do you explain that in spring the oceans emits lots of CO2 while there still is a lot of CO2 in the atmosphere? The same for vegetation in fall? Thus F = -M/RT?
Stallinga is completely wrong as he mentions the difference between RT and Tau, but throughout only uses the RT, while RT and Tau are caused by different, near completely independent, processes…
[…] New Study: Atmospheric CO2 Residence Time Is Only 5 Years – Too Short To ‘Affect The Climate’ […]
When they do carbon dioxide budgets based on fossil fuel emissions and the increase in atmospheric concentration increases, it seems only half the fossil fuel emissions show up in the atmosphere. In other words, half the CO2 is absorbed the year its emitted. The 5-10 years residence time makes sense in light of these measurements.
Residence time and concentration decay time are two different things. Humans emit around 1% of current CO2 into the atmosphere per annum. (4 ppmv of around 420 ppmv).
The increase in each year that is measured is around 2ppmv so around 2ppmv is absorbed by the oceans, life etc. At the current 420ppmv or so and if we assume 300ppmv was the “equilibrium value” absent significant human activity we get a time constant of 120/2 or 60 years. I’m willing to call it 40 to 60 years as I’ve seen such numbers elsewhere.
FWIW. I’m not worried at extra C02 in the atmosphere. 700ppmv might be nice.
Yes, and this is specifically addressed in the first half of the paper. https://www.mdpi.com/1099-4300/25/2/384
As the title indicates, the “decay time” is instead called the “adjustment time”.
The write-up didn’t emphasize this distinction due to a preference for brevity.
Kenneth,
Stallinga did mention the difference, but he used the residence time of a single CO2 molecule (about 4-5 years) as if it was the relaxation time (which is around 50 years)…
The residence time doesn’t change the total CO2 mass in the atmosphere, it is only an exchange rate between oceans, atmosphere and vegetation and the largest fluxes are even bidirectional over the seasons…
No Ferdinand, Peter Stellinga shows that the adjustment time can never be longer than the shortest of the residence times of the two reservoirs. He works this out with the use of a simple two-box first-order model.
No Frans, Peter Stalling is completely wrong here, as the RT is fully dependent of temperature changes and the adjustment time is fully dependent of the extra pressure of CO2 in the atmosphere above the temperature dependent dynamic equilibrium with the ocean surface.
Two completely independent processes without any influence of the RT on the residence time…
The last paragraph of course must be:
Two completely independent processes without any influence of the RT on the relaxation time…
If the eqiilibrium partial pressure of CO2 in the presence of land and water is 280 ppm, as we have risen to 425ppm, more and more CO2 should be absorbed. There should be less proportion staying in the atmosphere. Should we not see that by now?
The quantum is increasing as production rises, but the ratio of retained:produced should decline. Are we seeing it?
Perhaps our estimates of production are too crude to see this.
This confirms Salby, Berry, and Harde and falsifies the notion that reducing our emissions will reduce atmospheric CO2 meaningfully. Most of the recent rise in atmospheric CO2 is natural and a result of warming not the cause o warming.
Salby and Berry are science frauds
If Harde agrees with them, then he is a science fraud too
Manmade CO2 accounts for 33% of atmospheric CO2
Not 3% or 5%.
Only stupid people make that false claim.
Once again Richard Greene offers nothing other than name-calling as his “rebuttal”. He claimed he was not going to be commenting here anymore. And yet here he is, again, with the childish smearing attempts.
Why should anyone take anything he writes seriously?
I was tempted to respond to this ridiculous article, with some basic climate science facts which you and some readers deny, opposing an estimated 99.9% of scientists in the world, including “skeptic” scientists such as Willam Happer and Richard Lindzen.
You appear to be in the 0.1% along with Salby and Berry, making you a science denier — and science deniers are completely ineffective in refuting CAGW because they appear to claim there is no greenhouse effect and CO2 is not part of it. Total science denying of the worst kind.
If you can not even admit why CO2 levels increased by +50% since 1850, then you are clueless about climate science.
Manmade CO2 emissions were in the +250ppm to +300ppm range since 1850
Actual atmospheric CO2 increased by +140ppm,
from an estimated 280ppm in 1850 to a measured 420ppm in 2023
Answer these two questions to refute 99.9% of scientists in the world, Mr. Smarty Pants… (and I know in advance that you will fail because everyone has failed in the past five years that I have asked these two simple questions to conservative science deniers, who usually just ignore the questions, because they are dumbfounded:
Question !
If the +250ppm to +300ppm of manmade CO2 emissions since 1850 did not increase the atmospheric CO2 by +140ppm, after nature (clouds, land and plants) absorbed some, then where did that +250ppm to +300ppm go?
Question 2
If manmade CO2 emissions did not increase the atmospheric CO2 level by +140ppm since 1850, then what did?
Both questions require logical answers to refute the 99.9% consensus in the world that manmade CO2 emissions increased atmospheric CO2 about +50% since 1850
I stop at this website because Pierre writes good article and I often recommend them on my own blog. I have even recommended one of your articles. You got lucky. Not this one, of course.
The Richard vs. Richard war continues
Richard Greene
Bingham farms, Michigan
https://honestclimatescience.blogspot.com/
Two questions that you must answer, but won’t be able to, so you will ignore them and perhaps launch another character attack rather than answers:
The estimated +250ppm to +300ppm of manmade CO2 emissions went into the troposphere since 1850
The atmospheric CO2 level increased by about +140ppm since 1850
(1) If the +250ppm to ++30ppm of manmade CO2 emissions did not cause the +140ppm of CO2 increase, than what did?
(2) If the atmospheric CO2 level is really from 95% to 97% “natural, then where did the +250ppm to 300ppm of manmade CO2 emissions go to?
Come on now, the scientific answers should be easy if you are sure manmade CO2 emissions account for only 3% to 55 of the current 420ppm CO2 level.
3% of 420ppm CO2 would be 12.6ppm of CO2
5% of 420ppm CO2 would be 21ppm of CO2
I will be waiting patiently for your scientific answers.
maybe consult with Ed Berry or Murray Salby first?
Richard,
You are right, but please, calm down, that doesn’t help your case. Moreover, Murray Salby passed away last year and can’t be consulted anymore…
Ed Berry is another case, have commented several times on his blog to no avail…
His main error: he used the residence time upside down, which you may do if and only if all CO2 fluxes are unidirectional, which is certainly not the case for the seasonal fluxes which switch direction half the year…
As a “climate denier”, I get frustrated by these , in my view, idiotic questions about CO2 “residency” in the atmosphere. CO2 is unquestionably vital for life. Without it, the life is impossible. Since the life exists continually for millions of years that proves the CO2 existed continually as well. CO2 is recycled non stop. Plants take it up as “food” and natural decaying process, animals breathing it out (including humans) or out gassing from the oceans replenishes it, and the cycle repeats itself for ever. So could someone please explain to me, if CO2 is continually recycled, how can anyone determine that some molecule which came from a coal fired power station or car’s exhaust, can remind in the atmosphere for X amount of time, and why is this even a question? Plants don’t give two hoots where the CO2 came from. The fact is, CO2 is ALWAYS in the atmosphere, no matter where it came from. So why would anyone waste time and resources on something so silly, since CO2 is not even warming the Earth anyhow significantly! I am happy to be corrected, if some scientist can explain it.
[…] From NoTricksZone […]
lol. Science has already proven that the average energy in a molecule falling back to a ground state of electrical excitement is NOT consistent with the CO2 / ghg dribble.
1 Mole of air has “x” total electrons in that mole of air. This equates to 288 Kelvin.
An electrician worked it out. It is about time that supposed smart people figured it out. For goodness sake peeps.
Complete nonsense.
If the time manmade CO2 emissions stay in the atmosphere is only five years, then the increase in atmospheric CO2 since 1850, which was about 280ppm, would be about 2.5ppm a year times five years, or +12.5ppm in the past five years.
The current CO2 level would be 280ppm +12.5ppm (last five years), or 292.5ppm
But it seems the current CO2 level is 420ppm, not 292.5ppm
So the five-year claim must be total BS.
They have more lies than you have truths. The facts doesn’t mean anything anymore.
The article is wrong, for multiple reasons.
1. The MINOR reason it is wrong is that residence time is precisely determined from the decay rate of the 14C “bomb spike” after the atmospheric test ban treaty. This is a log scale plot of the decline of 14C levels in the atmosphere:
https://sealevel.info/logc14_two_half-lives.png
When atmospheric tests of A-bombs and H-bombs suddenly ceased (because of the atmospheric test ban treaty), the 14C concentration dropped on a near-perfect exponential decay curve, with a half-life of 11.5 years, implying a residence time of 16.6 years. (Note: ¹⁴CO2 is 4.5% heavier than normal ¹²CO2, which affects biological uptake and diffusion rates slightly. But not much.)
2. The MAJOR reason it is wrong is because it confuses “residence time” with “adjustment time” (a/k/a “effective residence time”).
The “residence time” is implied by the percentage of isotopically identifiable carbon remaining in the atmosphere, some period after a perturbation of its level. But it is much shorter than the “effective residence time” or “adjustment time,” for a change in the total amount of carbon in the atmosphere.
The reason is that some of the processes which remove ¹⁴CO2 from the atmosphere do so by exchanging it, one-for-one, for ¹²CO2. Those processes cause the fraction of “fossil carbon” in the atmosphere to decline without actually reducing the amount of CO2 in the atmosphere. That means the 11.5 year half-life and 16.6 year residence time are necessarily less than the effective lifetime of CO2 emissions.
The effective lifetime of anthropogenic additions to CO2 in the atmosphere, defined as the time it would take for (1-(1/e)) = 63% (sometimes rounded to 2/3) is roughly fifty years, making the half-life about 35 years.
That’s the result that Prof. Richard Lindzen reported during the Q&A (3rd video) of this (excellent!!!) lecture:
● Part 1:
https://www.youtube.com/watch?v=hRAzbfqydoY
● Part 2:
https://www.youtube.com/watch?v=V-vIhTNqKCw
● The Q&A which followed:
https://www.youtube.com/watch?v=69kmPGDh1Gs (including his discussion of CO2 atmospheric lifetime)
That’s also the approximate result that Dr. Roy Spencer found:
http://www.drroyspencer.com/2019/04/a-simple-model-of-the-atmospheric-co2-budget/
That’s also the approximate result which I got, first with a little program to simulate declining CO2 levels, based on the historical CO2 removal rate as a function of CO2 level, and then with a modified version of the program based on Dr. Spencer’s model. The source code is here:
https://sealevel.info/CO2_Residence_Times/
Ferdinand Engelbeen reported roughly the same result, here:
https://edberry.com/blog/climate-physics/agw-hypothesis/contradictions-to-ipccs-climate-change-theory/#comment-50170
3. We have solid data which proves beyond legitimate dispute that mankind can claim credit for all of the 105 ppmv increase in atmospheric CO2 concentration since precise atmospheric CO2 concentration measurements began in 1958:
https://sealevel.info/carbon/carbonflux.html
The mistake which the climate alarmists make is in their claim that the increase in atmospheric CO2 is harmful, or even a “crisis” or “emergency.” Actually, the scientific evidence is compelling that man-made climate change is modest and benign, and CO2 emissions are beneficial, rather than harmful.
Scientists call the periods of warmest climate “climate optimums,” including periods like the Eemian climate optimum, which is believed to have been several degrees warmer than now.
The supposed major harms are all merely hypothetical (& mostly implausible). None of them are actually happening:
* Sea-level rise has not significantly accelerated.
* Storms have not worsened.
* Droughts have not worsened, and drought impacts have been significantly mitigated by higher CO2 levels.
* Floods have not detectably worsened.
* Fires have not worsened.
* Corals and polar bears are doing fine.
The benefits of rising CO2 levels are real, measured & very large: improving crop yields, and a greening planet.
The benefits of CO2 for agriculture have been settled science for over a century. Note the date on this article!
Gradenwitz A. Carbonic Acid Gas to Fertilize the Air. Scientific American, November 27, 1920.
doi:10.1038/scientificamerican11271920-549
https://sealevel.info/ScientificAmerican_1920-11-27_CO2_fertilization.html
This NASA video is about how CO2 is greening the Earth:
https://www.youtube.com/watch?v=zOwHT8yS1XI
You can learn more, and find documentation for the facts I’ve mentioned, here:
https://sealevel.info/learnmore.html
I want to thank Dave Burton for writing a brilliant article, with trusted (by me) sources disguised as a comment.
I read about 24 climate and energy articles every morning and some of the comments too. This may be the best comment I’ve read so far this year.
I particularly like the mention that more CO2 is not bad news.
I have been advocating since 1997 for double to triple the current CO2 level to improve growth of C3 plants (85%). make winter nights slightly warmer in colder nations, and most important: Causing women to wear smaller bikinis on the beach to beat the heat, which is the subject of 975 of my climate research.
Thanks for the clarification.
Points for further clarification:
1) “a stable” CO2 content right now is supposed to be 280 ppm? Natural production and absorption cycles if no human addition would leave the world at 280 ppm? That’s the stable partial pressure between the oceans and the atmosphere right now?
2) 50 years is how long ANY CO2 introduced into the atmosphere above 280 ppm stays there? Or is there an exponential tail of some amount that hangs out for 100 years and is added to every year?
3) a time plot of CO2 additional input (as a theoretical ppm) vs measured additional ppm shows a consistent, declining or rising slope, indicating planetary absorption adjustment or saturation?
Hi Douglas,
Those are good questions. Here are my best efforts at answering them.
1. It’s probably closer to 295 ppmv, now. It used to be about 280 ppmv, but anthropogenic CO2 emissions have slightly increased the amount of carbon in other carbon reservoirs, such as soils and oceans, so the hypothetical equilibrium level would be slightly higher, now, than it was in the late 1700s (when it was 280 ppmv).
2. There is, indeed, a very long exponential tail in the hypothetical CO2 concentration decay curve. 35 years is the approximate length of the first half-life, but subsequent half-lives would be longer. That’s because some of the processes which move carbon between “reservoirs” are very, very slow. E.g., the AMOC overturning times are on the order of a thousand years.
Climate activists frequently claim that anthropogenic CO2 has a residence time of “centuries” or even longer. Such claims are based on integrating the “long tail” of its theoretical decay curve.
That’s a very silly thing to do, because the long tail would only become relevant if CO2 levels were below 340 ppmv, in which case we’d be suffering from a much more severe CO2 deficit, and more CO2 would be the opposite of a “cost.”
They do that to inflate their their “social cost of carbon” estimates. But that is remarkably silly, because it means their counting CO2 as harmful even when CO2 levels were below 350 ppmv. Even if you don’t agree with me that CO2 increases above 420 ppmv are beneficial now, nobody of consequence thinks CO2 level increases were harmful when CO2 levels were below 350 ppmv.
3. The natural CO2 absorption / removal rate is rising, rapidly.
That should not surprise you, if you realize that most of the processes remove CO2 from the air run at rates which are linearly related to the atmospheric CO2 concentration. That is true of the rate of CO2 absorption by water, it is also true of the rate of CO2 uptake by trees and other C3 plants (up to around 1000 ppmv), and it true of the rate of CO2 removals by rock weathering.
Each increase in atmospheric CO2 concentration by 43 ppmv (from Dr. Roy Spencer’s calculations), or 50 ppmv (from AR6 WG1 Table 5.1 numbers), accelerates the net rate of natural CO2 removals by about 1 ppmv per year.
Refs:
https://sealevel.info/AR6_WG1_Table_5.1_annot1.png
https://sealevel.info/AR6_WG1_Table_5.1_removal_rates_acceleration_vs_CO2_level.txt
Douglas,
I see that David Burton has done all the work…
Only a small remark:
The IPCC uses the Bern and similar models to investigate the long tails of residual CO2 with a relative huge part retained for many centuries.
That is right only for the ocean surface, which saturates at 10% of the change in the atmosphere. That is called the Revelle/buffer factor.
That doesn’t count for vegetation, where all trees and most other plants (of the C3-cycle) have their optimum growth at about 1500 ppmv…
That doesn’t count for the deep oceans, which are highly undersaturated for CO2 at the cold ocean sinks near the poles, which sink into the deep oceans, mainly in the N.E. Atlantic.
The only problem is that it takes a lot of time, because of the limited exchange between the atmosphere and the deep oceans.
On the other side: when everything is in equilibrium, all human emissions up to now (about 400 PgC) are only 1% of what already resides in the deep oceans (estimate: 37,000 PgC). That may give about 1% increase in the atmosphere or only 3 ppmv…
[…] *** New Study: Atmospheric CO2 Residence Time is only 5 Years – Too Short to ‘Affect the Climate’ […]
I have been following the poster TdeF over at Jo Nova’s site for quite a while and have a great deal of respect for his scientific knowledge and analytical ability.
He has done a series of posts on the half life and residence time of CO2 in the atmosphere and shows how it is not possible for the increase to be 100% due to human causes due to the C14 measured levels from circa 1958 to now.
I asked him to review the posts above and for his comments, which I have copied below.
I have not done this to create a spat between posters as I have a genuine interest in the issue as it goes to one of the fundamental premises of the CAGW believers.
I also not been convinced that man has been the sole contributor to the increase in CO2 as there are way too many natural sources that can alter the outcome.
From this research https://scientiamag.org/c-14-from-nuclear-bomb-tests-found-in-the-deep-ocean-trenches/
We know that C14 has crossed to the ocean depths so the boundary layer concept does not hold.
From TdeF
He then tries to expand the half life or residence time massively and prove everyone wrong. I quoted previously from about 18 published papers that the calculated half life in many papers (not residence time which is 40% more) is about 5 years. Residence time 8-10 years.
Surely he should have paused to consider that he is not just saying one researcher is wrong, but 18 of them and I am sure there are many more.
To preface this explanation, half life is self evidently the time to half the amount or 1/2.
Residence Time is 40% longer than half life and is the time to 1/e or 1/2.78 rather than 1/2 or half.
(technically residence time is the reciprocal of k so Tr = 1/k. e-kt = e-t/Tr).
Scientists like to use 1/e in publications rather than half life simply because it makes their calculations simpler.
(No one uses logs to the base 2. 2-t/thalf is the equivalent expression but logs come in base 10 and base e, then natural logarithm, not 2.)
So apart from the difference of 40%, they are the same thing. I find half life an easier concept to explain.
So where exactly is he wrong?
1. We have a perfect exponential curve of half life of 11.5 years. Agreed.
1/k value (‘Residence time’) of 16.6 years. Agreed.
If you even look at the graph and ask for half life for C14 in the atmosphere, the time to drop to half that you visually determine around 12.5 years. So far agreed.
And you might conclude this 16.6 years is the residence time and half life of CO2 in the atmosphere 40% less but here he is wrong. He has the residence time of C14 not CO2.
2. Most CO2 molecules are made with C12. But in the air thanks to the atom bomb tests you had 2 C14 molecules every 1 trillion CO2 molecules.
So what you are measuring is the loss of C14, not the rate at which CO2 is exchanged with the water which is what we are trying to estimate.
But in the water there is already the equilibrium 1 molecule of C14 for every 1 trillion CO2 molecules.
So for every two atmospheric CO2 molecules which enters the water, two come out with the CO2 exchange.
But the concentration of C14 molecules in the atmospheric CO2 is double at 2 in 1 trillion
So for every two C14’s molecules absorbed from the air, one 1 C14 molecule from the water comes out. Halving the apparent absorption of C14! So effective half life of C14 in the air is twice as long as the CO2 half life.
because you have to exchange two CO2 molecules to remove one C14 tagged molecule.
So the half life for CO2 molecules to be replaced by ocean CO2 molecules is only 5.75 years.
Then how did he calculate 50 years ‘effective lifetime’ from 16.6 years? He used a linear fraction, a simple ratio equating the balance left after the residence time as (1-(1/e)) = 63%. So 37% he equated to 16.6 years. So using a linear ratio he has come up with 44.8 years or as he calls it, 50 years. But it’s not right because you cannot treat and scale an exponential decay as a straight line! The decay never stops. It just halves and halves.
The big mistake is a factor of 2 in half life and then this is compounded, not scaled. Random radioactive decay is such that the amount lost is directly proportional to how much is left. So you use half lives. And three half lives means 1/2*1/2*1/2 or 1/8th. After 15 years you are down to 12.5%.
So for CO2 (not C14) half of all CO2 and so also half the fossil fuel CO2 goes into the ocean in 5 years.
And it keeps vanishing..
3/4 gone in 10 years 75%
7/8 gone in 15 years 88%
15/16 gone in 20 years 94%
The fossil fuel CO2 is 94% in the ocean in 20 years.
And this excess fossil fuel CO2 above the long term equilibrium amount is distributed in the ratio was already observed and controlled by Henry’s Law, 98% in the ocean and only 2% in the air.
Now the IPCC gives the HALF LIFE (without proof or reference I could find) at 80 years as the gold standard in the 4th IPCC report. And all Greenhouse gases are scaled to CO2, so it’s all a mess. At best what he has calculated is some sort of time it to be all gone after 50 years, still far less than the IPCC published half life of 80 years.
I also note that the papers claim that fossil fuel CO2 is 15%. It isn’t. G.J.Fergusson in 1958 calculated 2.03%+/-0.15%. It is now slightly higher at 3.0%. The very high rate of absorption of CO2 means the extra fossil fuel CO2 does not hang around. Which is a shame
And it is easy to overlook that you are measuring the permananent loss of long lived radioactive C14 from the entire biosphere. That is enough to show that the CO2 system is not closed, that the CO2 tagged with C14 leaves, never to return in the endless CO2 swapping process. And the ONLY way this is possible is for the CO2 to enter the IPCC Forbidden zone, the deep ocean. Something the Bern model shown in every diagram explicitly denies.
So the fact that C14 is going down fast proves the whole ocean is involved in recycling CO2. And that’s the end of the story of CO2 build up in the artificially small atmosphere + the first 100 metres of ocean, the way so called Climate scientists get around the rapid cleansing of the oceans, the total absorption of excess fossil fuel CO2.
The IPCC says it’s just too slow compared to a human lifetime. But the rapid fall without even understanding how it happened, the vanishing of C14 alone, even at first sight, says they are categorically wrong. The excess C14 tagged CO2 from the atom bomb blasts has complete vanished from the biosphere. And that means it is absorbed into the deep ocean where it is diluted by a factor of 50, 98:2
manmade CO2 emissions added from 250ppm to 300ppm of CO2 to the atmosphere since 1850
An estimate
Manmade CO2 emissions accounted for an estimated +250 to +300ppm of extra CO2, beyond the 280ppm CO2 estimated for 1850
Atmospheric CO2 increased by +140ppm since 1850
Also an estimate
What happened to the rest of the manmade CO2 emissions?
Theye were obviously absorbed by nature (oceans, land and plants)
No more needs to be known to make the following simple conclusions:
100% of the CO2 increase from 280ppm to 420ppm
is from manmade CO2 emissions
A lot of those manmade CO2 emissions
were absorbed by nature
There is no other logical explanation
for these results.
IT’S THAT SIMPLE
YOU ARE THINKING TOO MUCH.
The explanation is simple.
No one has provided an alternative explanation that makes sense
It makes sense that CO2 spewed in the atmosphere would increase the CO2 level in the atmosphere.
X Can anyone tell me why so many Climate Realists still believe only 3% to 5% of the 420ppm of atmospheric CO2 had manmade origins?
It appears to me that they are confusing the annual seasonal carbon cycle with net additions of CO2 to the atmosphere by manmade CO2 emissions.
Annual manmade CO2 emissions are a tiny percentage of total carbons flows during a year, but that comparison is meaningless. That’s my guess of what the science deniers believe.
Scott,
Your reasoning is the wrong way out:
“So for every two C14’s molecules absorbed from the air, one 1 C14 molecule from the water comes out. Halving the apparent absorption of C14! So effective half life of C14 in the air is twice as long as the CO2 half life.”
Because 2 molecules 14C go into the deep oceans and only one comes out, the decay rate of the 14CO2 bomb spike is much faster than the decay rate of a 12CO2 spike… Near three times faster. The apparent absorption of 14C is much faster than for 12CO2.
See the mass balance for the year 1960, at the maximum 14C level of the bomb tests:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/14co2_distri_1960.jpg
97.5% of all absorbed 12CO2 mass into the deep oceans returns the same year in the upwelling as a (different) 12CO2 mass. Only 45% of all 14CO2 returns the same year (including the radiative decay rate over ~1000 years).
Then you are mixing up two definitions: the residence time indeed is 4-5 years and you did mention it as:
“So the half life for CO2 molecules to be replaced by ocean CO2 molecules is only 5.75 years.”
Replaced is the keyword: the individual fossil fuel CO2 molecules are replaced by CO2 molecules from other reservoirs (oceans, vegetation), but that does NOT change the total amount of CO2 in the atmosphere with one gram.
Even if all fossil CO2 molecules were replaced with natural ones within a month, the total increase in CO2 mass still is the same and 100% caused by human emissions… Over the past 70 years the sum of all natural CO2 in and out fluxes was always negative: more sink than source, thus all increase is from the human contribution.
That increase of 415 ppmv CO2 above the current 295 ppmv dynamic equilibrium between ocean surface temperature and atmosphere is what drives extra CO2 into the oceans and that has a decay rate of around 50 years. That is the real decay rate, absolutely nothing to do with the residence time of 5 years. Thus TdeF is right.
Even with the huge exchange rate of 5 years, the current percentage fossil fuel CO2 in the atmosphere is already over 10%, as can be deduced from the huge drop in 13C/12C ratio over the past 170 years:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/sponges.jpg
The IPCC comes with much longer decay rates and very long tails based on the Bern and similar models, which assume a saturation of the different sinks. That is true for the ocean surface, but not for vegetation and absolutely not for the deep oceans. But the latter has a limited exchange rate with the atmosphere, so that needs far more time…
[…] Injustice”*** Report: Net Zero could cost Americans more than $50 trillion*** New Study: Atmospheric CO2 Residence Time is only 5 Years – Too Short to ‘Affect the Climate’A simple reason why net zero is impossibleThe Climate Emperor Is Now NakedCO2 Reality vs. Narratives […]
Scott,
Your reasoning is the wrong way out:
“So for every two C14’s molecules absorbed from the air, one 1 C14 molecule from the water comes out. Halving the apparent absorption of C14! So effective half life of C14 in the air is twice as long as the CO2 half life.”
Because 2 molecules 14C go into the deep oceans and only one comes out, the decay rate of the 14CO2 bomb spike is much faster than the decay rate of a 12CO2 spike… Near three times faster. The apparent absorption of 14C is much faster than for 12CO2.
See the mass balance for the year 1960, at the maximum 14C level of the bomb tests:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/14co2_distri_1960.jpg
97.5% of all absorbed 12CO2 mass into the deep oceans returns the same year in the upwelling as a (different) 12CO2 mass. Only 45% of all 14CO2 returns the same year (including the radiative decay rate over ~1000 years).
Then you are mixing up two definitions: the residence time indeed is 4-5 years and you did mention it as:
“So the half life for CO2 molecules to be replaced by ocean CO2 molecules is only 5.75 years.”
Replaced is the keyword: the individual fossil fuel CO2 molecules are replaced by CO2 molecules from other reservoirs (oceans, vegetation), but that does NOT change the total amount of CO2 in the atmosphere with one gram.
Even if all fossil CO2 molecules were replaced with natural ones within a month, the total increase in CO2 mass still is the same and 100% caused by human emissions… Over the past 70 years the sum of all natural CO2 in and out fluxes was always negative: more sink than source, thus all increase is from the human contribution.
That increase of 415 ppmv CO2 above the current 295 ppmv dynamic equilibrium between ocean surface temperature and atmosphere is what drives extra CO2 into the oceans and that has a decay rate of around 50 years. That is the real decay rate, absolutely nothing to do with the residence time of 5 years. Thus TdeF is right.
Even with the huge exchange rate of 5 years, the current percentage fossil fuel CO2 in the atmosphere is already over 10%, as can be deduced from the huge drop in 13C/12C ratio over the past 170 years:
http://www.ferdinand-engelbeen.be/klimaat/klim_img/sponges.jpg
The IPCC comes with much longer decay rates and very long tails based on the Bern and similar models, which assume a saturation of the different sinks. That is true for the ocean surface, but not for vegetation and absolutely not for the deep oceans. But the latter has a limited exchange rate with the atmosphere, so that needs far more time…
Hi Ferdinand,
Thanks for responding.
So if I boil your argument down to actual data it is the C12/C13 ratio rather than C14 level that indicates FF use.
The rest is really an assumption about sinks versus sources as we can only measure one aspect of that equation and that is human emissions, everything else is not measured and surprising sources keep getting found.
https://joannenova.com.au/2019/06/new-finding-phytoplankton-are-much-bigger-players-in-co2-levels-than-realized/
We know that oxygen is transported to the deep ocean and therefore so is CO2 every year through the process of cold water settling and flushed to other areas with ocean currents. so the boundary saturation assumption is not true.
https://eos.org/articles/oxygen-levels-measured-in-a-lung-of-the-deep-ocean
So if the C12/C13 is the indicator it was falling massively before human CO2 emissions became anything more than background noise, which suggests another driver of the C12/13 ratio change.
I also remember seeing a lot of discussion around the C12/C13 ratio being used and it certainly was not a settled measure.
I will as TdeF to have a look at your response.
Thanks again
Hi Scott, did saw that only today…
There are two items known in the carbon balance: human emissions (fossil fuel sales – taxes) and the increase in the atmosphere.
In the past 60+ years, the increase in the atmosphere was always smaller than human emissions, with only two borderline (El Niño) years.
If we look at the carbon balance:
increase in the atmosphere = human emissions – human sinks + natural emissions – natural sinks
or app.:
4.5 PgC/year = 9 PgC/year – 0 + X – Y
or
X – Y = -4.5 PgC/year
Even without knowing any individual natural carbon flux we know within narrow borders the net effect of all natural ins and outs together. The absolute height of X and Y doesn’t play any role, neither that some individual natural emission (volcanoes) doubled or tripled in one year: the net effect is known and that was negative in all years:
If X = 10 PgC/year then Y = 14.5 PgC/year
If X = 100 PgC/year then Y = 104.5 PgC/year
If X = 1000 PgC/year then Y = 1004.5 PgC/year
The remarkable point is that in the past 60+ years, the natural variability even for the extremes (Pinatubo, El Niño) is not more than +/- 1.5 ppmv around the trend. The latter is around 90 ppmv in the same period…
[…] Link: https://notrickszone.com/2023/03/23/new-study-atmospheric-co2-residence-time-is-only-5-years-too-sho… […]
Wonderful post