More on Sulfur Dioxide
By Ed Caryl
Nearly all of the literature about SO2 claims that it is not a greenhouse gas. This “consensus” opinion is based on the belief that SO2 residence time in the atmosphere is too short for the gas to have an effect.
In my last article on this subject it was asserted that the severe pollution from Norilsk in Siberian Russia moves out over the Arctic Ocean on the prevailing winds, preventing cooling, slowing the freeze-up. This is seen on the anomaly maps as warming. (It is now warmer than the 1998-2006 average.) As can be seen in Figure 1, the same thing happens in Arctic Canada, due to the SO2 from Canadian oil and gas fields that produce similar amounts of SO2.
There have been several estimates for the residence time of SO2 in the atmosphere. Keep in mind that residence time is the half-life of the substance in the air, that is, the time it takes for half of the material to disappear. If the residence time of SO2 is ten days, this means that, ignoring dilution, the 50,000 parts per million present at Norilsk, will be 25,000 parts per million 10 days later as the wind pushes that parcel of air over the Arctic Ocean, 12,500 parts per million after 20 days, 6,250 after a month, about 750 ppm after two months, etc. Because of inversions over the Arctic Ocean, there isn’t much vertical mixing to dilute the gas, so most of the mixing is horizontal in about a 100 meter altitude layer.
But, (and it is a big but) the residence time increases with a reduction in temperature. In the Arctic, at temperatures way below zero centigrade, the residence time may be more than 30 days, so multiply the time intervals above by three. That covers a whole season. And the SO2 is continuously being replenished. We are not describing an impulse in time like a volcanic eruption. Concentrations over the Arctic Ocean may build up and approach the concentrations at the source, if the wind blows steadily from south to north for an extended time. The residence time increases if there is nothing to remove the SO2.
Here are the things that remove SO2 from the atmosphere (read here).
1. Direct photo-oxidation (in the presence of oxygen and water).
But in the Arctic in the winter there is no sunlight, so that doesn’t function. The water vapor concentration is also very low. Below freezing, water vapor partial pressure is below 5 mbar; at -20°C it is below 1.5 mbar (below 0.5% and 0.15% of the atmosphere).
2. Indirect photo-oxidation (with O2, NOx, OH, organic oxidants).
Same problem as 1, no sunlight. And no sunlight to produce OH radicals.
3. Oxidation in liquid droplets (in the presence of liquid water).
There is no liquid water remember? The temperatures are way below zero.
4. Homogeneous aqueous phase oxidation.
Same problem as 3.
5, Catalyzed oxidation in liquid droplets (requires metal ions as well as water droplets).
This one might work, as there are lots of metal ions in the pollution from Norilsk, if there are super-cooled water droplets. But as in 1, the water vapor availability is very low because of the cold. What acid production that does occur happens in the smelter smokestacks where water vapor and temperatures allow 3, 4, or 5 to operate. Once the SO2 gets into the cold, dry, arctic air, all that stops.
6. Catalyzed oxidation on dry surfaces.
No joy here. Once it reaches the coast, the stuff is over open ocean or ice.
So, in winter, once SO2 leaves the smelter smokestacks, there is no mechanism to get rid of it. No wonder the Arctic is warming!
Is this what happens? Is the Arctic warming in winter? The climate scientists say it is, and in this regard they are correct.
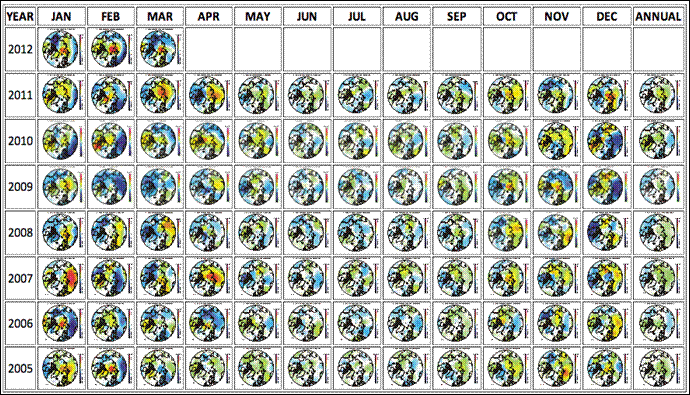
Figure 2: Monthly maps of Arctic temperature anomaly for the last 7+ years. Source: Climate4You.
Note in Figure 2 the amount of red and orange (warm) areas in the October through April months. December and January have the most red in several of the years above. Most of the warm areas are right over Norilsk in Arctic Siberia. The warming happens only in the cold months. In Arctic summer there are mostly cool colors. In Arctic summer the humidity rises, sunlight produces OH radicals, and all the mechanisms 1 through 6 above begin operating, removing the SO2.
According to one source, 93.5% of the SO2 emissions in 1998 were in the Northern Hemisphere. The Northern Hemisphere is where most of the measured warming is taking place. Circumstantial evidence would seem to point to SO2 as the culprit. Can SO2 act as a greenhouse gas? It has an infrared spectrum with two peaks at 7.27 and 7.345 µm. This is on the shoulder of one of water vapor’s absorbance peaks. In the absence of water vapor, in the Arctic cold, it can replace it as a greenhouse gas. In that role it can produce back radiation just as water vapor, CO2, and methane do. In past periods of high volcanism high volcanic emissions of SO2 have overwhelmed natural reduction mechanisms and have produced warming periods (see here).
The study of SO2, especially how it acts in the polar regions, has been neglected in favor of pushing CO2 as the ONLY cause of global warming. As is easily seen by looking at anomaly maps, the warming is not global. It is concentrated in the Arctic at certain locations, and to a lesser degree, the Antarctic, again, in the Southern Hemisphere winter. In the Antarctic, warming seems to be concentrated around the large research stations. All the stations in the Antarctic burn oil for heat, power, and transport. Is SO2 and the Antarctic winter the problem there?





Now we know how Hansen is pulling “global” warming out of his hat. How much would the local area cool if the smelting operations were shut down? 2°C? 3°C? 5°C?
Ed,
You didn’t mention that SO2 condenses (boils) at -10°C…
http://en.wikipedia.org/wiki/Sulfur_dioxide
The boiling point depends on the partial pressure. That is also the reason that CO2 does not rain from the sky in Antarctica, where temperatures go below the boiling point of CO2 regularly.
DirkH,
I think you confused CO2 with SO2. They are different substances!
Liquid CO2 cannot exist under atmospheric pressure at any temperature, because its triple point is higher than 1 bar!
Physical properties of CO2
http://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Units=SI&Mask=4#Thermo-Phase
You may need to consider a phase diagram similar to Mollier’s diagram.
http://de.wikipedia.org/wiki/Mollier-h-x-Diagramm
I must say I haven’t performed any calculations. Not yet.
Okay, no liquid CO2. let me rephrase: The partial pressure is important. Otherwise CO2 would snow out of the sky in Antarctica. And no, I didn’t confuse CO2 and SO2. I used CO2 as an example. In other words: The partial pressure of SO2 even at Norilsk is far too low as to make it condense.
http://en.wikipedia.org/wiki/Clausius%E2%80%93Clapeyron_relation
No, DirkH.
The triple point of a substance is invariant – it means, it doesn’t depend on temperature or pressure, neither. Of course, the partial pressure plays a role. In this case it’s too low for CO2 resublimation, either.
Actually, there must be considered a ternary system composed of steam, SO2 and air. And don’t forget, SO2 reacts with steam that is always presented in the atmosphere.
(Now that THAT’s decided…)
Ed, what Temperature Dataset did you use?
The maps came from Climate4You. They use GISS.
Are there any direct measures of SO2 in the relevant areas that would falsify the hypothesis that duration is multiplied in the “cold wild” if they fell below predicted criteria?
I found one study done in the Canadian Arctic, in April-May, in one year. (I don’t remember which one, but it was after 1995.) They found traces of SO2, but the sun was already destroying it. No one likes to fly over the high Arctic in winter. It’s dark and very cold. The satellites need sunlight to get reflectance data, so all we have is IR temperature. There is one image of the SO2 plume from Norilsk, but it was done in summer and only showed the immediate region. There is too much preconceived notion that SO2 cools rather than warms. The subject cries out for more study. A big problem is that the area is northern Russia, closed to outsiders, and the Russians themselves have economic reasons not to release data or study it themselves.
In an unexpected spasm of half-honesty, Der Spiegel reports
“Weak sun caused cold period 2,800 years ago” (Homerian minimum)
http://www.spiegel.de/wissenschaft/natur/schwaechelnde-sonne-loeste-antike-kaelteperiode-aus-a-831401.html
cites proxy research by Achim Brauer, GFZ, sediments from Meerfelder Maar, a sea with volcanic origin in the Eiffel.
Article contains the usual caveats; influence of sun is small compared to what evil Western civilization does to climate; only regional changes etc; and some slurs against skeptics.
what’s about CH4 release in the arctics ??
Why don’t you clue us in to what the handlers of “Envi”sat want us to believe?
Currently the Arctic is a carbon sink.
http://nsidc.org/cryosphere/frozenground/methane.html
http://www.nature.com/ngeo/journal/v5/n5/full/ngeo1452.html
and references therein……..
“SpaceScience”, just throwing links around to a former scientific journal without hinting at what you want to say is impolite. I won’t follow the link to the warmist journal. You’re too arrogant to speak a single sentence to us lowly skeptics? Fine, then go away, talk to the wall.