Even NASA says it:
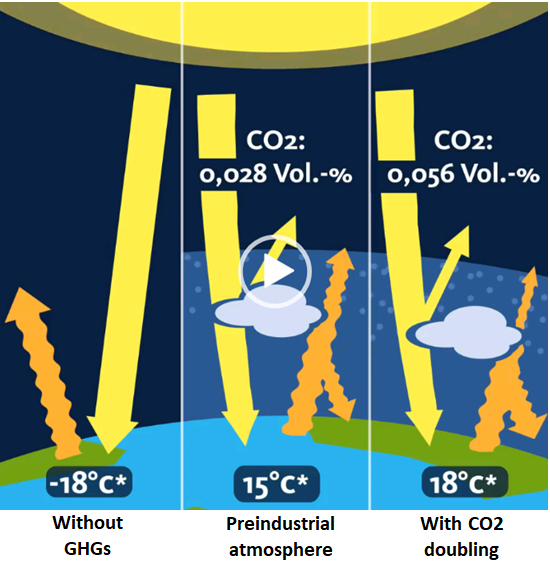
Without the Earth’s greenhouse gases (GHG) in the atmosphere, the planet would be on average a frigid -18°C.
But because of the preindustrial 280 ppmv CO2 and other GHGs in our atmosphere, the average temperature of the Earth thankfully moves up by 33°C to +15°C (see chart below), based on the Stefan Boltzmann Law.
Global warming theorists say the Earth’s surface preindustrial temperature was supposed to be 15°C. And today CO2 is supposed to have warmed it another degree, to 16°C. Image: www.klimamanifest.ch.
And because CO2 has since risen to about 410 ppmv today, the global temperature supposedly should now be about another 1°C warmer (assuming positive feedbacks) bringing the average earth’s temperature to 16°C.
And once the preindustrial level of CO2 gets doubled to 560 ppm, later near the end of this century, global warming alarmists insist the Earth’s temperature will be near 18°C, see chart above.
So we are now supposed to be at 16°C today and warming rapidly. But what is the globe’s real average temperature today? 15.8C? 16.0C? 16.5°C?
Answer: astonishingly the official institutes tell us it is only 14.7°C!
For example, data from the World Meteorological Organization (WMO) shows us the global absolute temperatures for the previous 5 years:

Image: www.klimamanifest.ch, data source: WMO in Geneva.
As the image above shows, the global absolute temperature last year was just 14.68°C.
This is 0.32°C COOLER than the 15°C we are supposed to have with 280 ppmv, and a whopping 1.32°C cooler than the 16°C it is supposed to be with the 410 ppmv CO2 we have in our atmosphere today.
So why are we missing over 1.3°C of heat? Why is there this huge discrepancy between scientists?
German scientists say Earth temperature was 15.5°C – in 1990!
In May, 1990, even the German government stated in its major report on climate (BT-DRS 11/8030, p. 29) that the global average temperature back then was 15.5°C and that the natural temperature was supposed to be 15°C — like NASA says. The German government reiterated that figure in 1992.
In 2003, renowned German climate scientist Prof. Mojib Latif also confirmed in his book that 15°C was the “optimum temperature” for the Earth.
And in his 2003 doctorate dissertation, Tim Staeger wrote that the natural Earth’s temperature without man-made impacts is “about 15°C”.
In fact, practically all German textbooks used at schools today say that the “natural temperature” of the Earth due to the greenhouse effects of the atmosphere is a “life-friendly average of 15°C” instead of -18°C.
PIK scientist: “15°C in 1850”
Moreover, Potsdam institute for Climate Impact Research (PIK) scientist Anders Levermann testified before the German Parliament last year, and confirmed that the global mean temperature of the Earth back in 1850 was 15°C, which means today it is supposed to be well over 16°C. So why is the WMO telling us it’s only 14.68°C and others like the PIK and NASA saying it’s about 16°C?
This massive discrepancy needs to be explained.
IPCC 2007 report in line with WMO
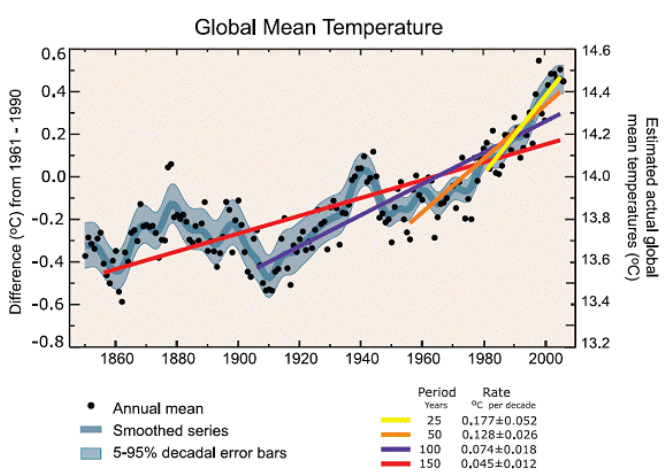
The IPCC indeed contradicts all the scientists and media who claim the global average temperature was 15°C back in 1850. Here’s the IPCC chart from the 2007 report:
How can 14.68°C be “too hot”?
As the chart above shows, the IPCC stated that the global mean temperature in 1850 was a relatively frigid 13.7°C – i.e. well below the “life-friendly” average of 15°C.
Today we are still below the 15°C, and so how can it be too hot?
Summary
There’s no doubt it’s gotten warmer since 1850, the peak of the Little Ice Age. But it’s clear nobody knows what the globe’s real average temperature is. Figures are being wildly tossed around. If we are to believe the IPCC’s 14.7°C figure, then we are still too cool and there is absolutely no warming crisis.
Scientists need to answer this question behind this huge discrepancy quickly. Would the real global temperature please stand up!
============================
Hat-tip: Das Klimamanifest von Heiligenroth





According to NASA/NOAA in 1997 it was 16.92C and even hotter in 1998.
They say that this changed due to a new baseline and new calculations.
see
https://www.ncdc.noaa.gov/sotc/global/199713
This is the last year that this “mistake” by NASA/NOAA will be available as it drops off the list of years that are available.
So take a screenprint now before it goes.
I say mistake because this is one of the very few years where they quoted the “actual temperature” rather than an anomaly.
[…] https://notrickszone.com/2019/10/19/controversy-swirls-as-numbers-dont-add-up-1-3c-missing-heat-eart… […]
Regulars of NTZ will know this, but for others:
The temperatures ought to be stated in Kelvin.
This would show how insignificant these are.
The multi-color chart “Global Mean Temperature” will do this if you replace °C (C°) with K.
Note also that it is necessary for the vertical scale (y-axis) to be expanded (exploded view) for the dots and lines to show as anything other than a smudge at the top of a very tall chart.
13.6°C = 286.75K
13.8°C = 286.95K
Albert Einstein had this to say about the idea that the atmosphere’s temperature is dominated by a 1-in-2500 molecule trace gas.
Albert Einstein, in his 1917 paper:
http://inspirehep.net/record/858448/files/eng.pdf
says this about radiative heating of a gas:
During absorption and emission of radiation there is also present a transfer of momentum to the molecules. This means that just the interaction of radiation and molecules leads to a velocity distribution of the latter. This must surely be the same as the velocity distribution which molecules acquire as the result of their mutual interaction by collisions, that is, it must coincide with the Maxwell distribution. We must require that the mean kinetic energy which a molecule per degree of freedom acquires in a Plank radiation field of temperature T be
kT / 2
this must be valid regardless of the nature of the molecules and independent of frequencies which the molecules absorb and emit.
He refuted the CAGW nonsense in advance. CO2 has no effect on atmospheric temperature.
This approach is most interesting. If they can’t agree on the “right” temperature for the planet how will they decide where to set it when they get control of the thermostat?
As it turns out their concept of a thermostat has been shown by good old data analysis to be falsified.
I would like to bring your attention to a new scientific analysis that is very good news. See
( https://www.youtube.com/watch?v=XfRBr7PEawY ) for radiosonde evidence that the atmosphere obeys ideal gas law and no greenhouse effect is present. It falsifies the greenhouse warming hypothesis that is being used as fact to support the claims of “climate crisis” that are frightening and demoralizing our children and youth.
So the the mechanism causing the “crisis” is itself missing and could not have been the cause of recent warming let alone any crises resulting from any warming that they might have measured no matter its cause.
Great questions to be answered.
Also most of the warming is above the 65N latitude, virtually no temperature recordings from that region in 1850.
First of all, the supposed science — including the global temperature “measurements” — discussed in this post is wrong, in fact physically incompetent. There is no valid global climate science, and no competent climate scientists, either alarmist or lukewarmers. Second of all, this post is not “news” at all.
I have pointed out the numbers “controversy” discussed above for many years, since my Venus/Earth temperatures comparison, that precisely confirmed the Standard Atmosphere model for Earth’s troposphere, and UTTERLY DISPROVED the so-called “greenhouse effect”, of a global warming due to increase in atmospheric carbon dioxide (or any other “greenhouse gas”, for that matter). That Venus/Earth comparison is the definitive evidence for correcting climate science on a whole handful of fundamental physical points, including how the atmosphere is warmed, what governs the global mean surface temperature, and just as importantly, what DOES NOT govern it (spoiler alert — none of the physical variables, that the climate scientists believe are causative of global temperature change, have any such global effect — and their physics cause-and-effect is precisely the reverse of what they believe and promulgate).
As I wrote back in 2013, on my blog, theendofthemystery:
“…I was amused by the fact that some scientists were saying (as if it were common knowledge) that the global mean surface temperature (GMST) was around 14.7°C, when for a century the Standard Atmosphere has given that temperature as 15°C–higher than the supposed current temperature,
despite a century of supposed warming. (However, lately I have seen efforts being made to counter my miniscule but definitive ridicule, with the inner cadre of “consensus” climate scientists claiming the GMST is 15.7°C. So I know they are listening to me, and working hard to stay ahead of the ridicule they so rightly deserve. But the 15.7°C claim is still at odds with the definitive(!) Venus/Earth comparison, because the latter confirms 15°C, not 15.7–that is how precise the comparison is.”
There has been NO global warming, certainly since the development of the Standard Atmosphere model of Earth’s troposphere over a century ago. The Earth was NOT cooler in 1850; the global mean surface temperature is VERY stable, against either global warming OR cooling (which, by the way but even more important than the present “climate debates”, means there have been no naturally-occurring global ice ages in Earth’s distant past — the earth and life sciences are all founded upon false premise, i.e. a false paradigm of undirected evolution of everything we observe in the world).
So why is the surface of Venus so hot? Is it atmospheric pressure or something else?
It’s gravity .
Pressure , density * velocity , is an effect not a cause .
It’s not gravity. How could it be?
Gas Laws. Gravity creates the Pressure – P that determines Temperature
PV = nRT
Avogadro,
You got it backwards. Temperature determines Pressure. Joule’s 2nd law.
Zoe says:
You got it backwards. Temperature determines Pressure. Joule’s 2nd law.
This is wrong! Gravity acts on the Mass, not on Temperature.
Mass does not change with temperature.
Gravity is the main factor that affects pressure through the mass. Venus has not only a thicker atmosphere, but also much more mass congregated.
@Zoe Phin 24. October 2019 at 6:01 AM
We are talking about an “adiabatic” process, where no heat is added (or removed) to the system. If you compress that gas, it’s temperature will rise. If you increase it’s volume, it will cool.
But changing it’s temperature by adding heat will cause it’s pressure to increase (if it can’t expand), or will increase it’s volume (decrease its density – think hot air balloon). It’s college freshman chemistry.
It’s a bit tricky, because the equation applies whether or not you add heat, but the final result depends on your knowing what the energy history of the gas is. If you don’t, you’ll probably get the wrong result, as you have in this case.
‘… the “natural temperature” of the Earth due to the greenhouse effects of the atmosphere is a “life-friendly average of 15°C” instead of -18°C …’.
As if being born in one of those relatively rare warmer episodes of the past half million years is not good enough for some people:
https://i0.wp.com/www.appinsys.com/globalwarming/GW_Part1_PreHistoricalRecord_files/image023.gif
No, they want to set the thermostat to an “ideal” 15C.
Gaia is a b1tch, but I wouldn’t entirely blame Her if She threw a tantrum and decided to give those ungrateful Earthlings something to really complain about by bringing forward the next major glaciation.
“This massive discrepancy needs to be explained.”
My understanding is that 15°C was a GUESS for SEA-LEVEL temperature made in the 1950s before all the data was available.
So why is the surface of Venus so hot? Is it atmospheric pressure or something else?
https://motls.blogspot.com/2010/05/hyperventilating-on-venus.html
Yonason, it can’t be due to pressure. It’s something else.
It isn’t due to pressure alone. The energy entering the system is distributed vertically throughout the atmosphere by gravity: the lower in the atmosphere, the higher the pressure; and the higher pressure, the higher the temperature.
See also here…
https://motls.blogspot.com/2010/05/venus-chris-colose-vs-steve-goddard.html
“…about 50-55 km above the surface of Venus, the temperature as well as pressure are close to the values we know from the Earth’s surface: around 1 atmosphere and approximately 0-50 °C.
…. [BUT] ….
…beneath this Earth-like layer, you still have 50-55 km of extra “air”. Because the basic laws of adiabatic heating (without heat transfer) – …. – imply that the temperature gradient [the “lapse rate”] is around 8 °C per kilometer in average, what a shock that the Venus’ surface will be found to be 400 °C warmer than what humans like to experience on the Earth. Recall that 50 times 8 equals 400.“
Yonason,
I think you have it backwards. It’s the temperature that creates atmospheric pressure. Joule’s 2nd law. It is the the temperature that outgasses molecules for gravity to work upon, not vice versa.
In thermodynamics, you start with hot and go to cold. It doesn’t get warmer as you descend, it gets colder as you descend.
Venus has a huge amount of geothermal energy, and that’s why it’s hot, and why it has a huge atmosphere.
@Zoe Phin
As I wrote above…
https://notrickszone.com/2019/10/19/controversy-swirls-as-numbers-dont-add-up-1-3c-missing-heat-earth-supposed-to-be-16c-but-its-only-14-68c/comment-page-1/?unapproved=1302973&moderation-hash=ddc0f208140283a155f463edcc31bf38#comment-1302973
And, of course it will cool if no heat is added, but we are talking about how that heat, once added, becomes distributed through the system.
This video may help you visualize adiabatic atmospheric processes.
https://www.youtube.com/watch?v=ObnWb7yspxA
Enjoy.
Yonason,
Wouldn’t the hydrostatic equilibrium as in our atmosphere preclude any net compression and expansion. Isn’t outward expabsion = inward compression?
More text at the bottom. Sorry I posted it out of thread.
The +33°C fairy tail is a total nonsense invented by dishonest and incompetent pseudo-scientists who apply laws (here, the Stefan-Boltzmann law and the Black-Body model) without understanding them and elsewhere than in their valid application field.
In 1972, the NASA found that the Moon mean temperature is approx. 40 K higher than predicted by the Stefan-Boltzmann law :
http://tech-know-group.com/papers/Greenhouse_Effect_on_the_Moon.pdf
Is there a greenhouse effect on the Moon too ?
According to the climate clowns, yes.
“Without the Earth’s greenhouse gases (GHG) in the atmosphere, the planet would be on average a frigid -18°C.” “The +33°C fairy tail is a total nonsense invented by dishonest and incompetent pseudo-scientists “.
Indeed. It requires a very special sort of clouds, that reflect some of the sunlight but do not interact with the upward infrared.
The real figure would be around 15K. So the combined effect of the GHG’s are only 15K and not 33K.
The story has been told so many times that even peoble who should know better believe it. How dare you.
CO2 is NOT the climate “control knob.”
Patrick Moore takes a sledge hammer to the garbage being spewed by climate activists. (Notice that I do not call the “scientists,” for very good reason.)
https://youtu.be/2kIcFIofUHk?t=272
Without CO2 we all die. Either the activists are too stupid not to know that, or they are too evil to care. Either way it’s not good.
The 33c mime is nonsensical . The most fundamental value for any orbit is the ` grayBody temperature which for our orbit is ~ 278.6 +- 2.3 from peri- to ap- helion .
That is the value upon which all further elaborations must be computed , including the step-function spectrum which produces the 33c meme .
http://cosy.com/Science/AGWpptHypotheticalSpectra.jpg
https://www.americanthinker.com/articles/2012/11/fourteen_is_the_new_fifteen.html
Phil Jones:
https://agupubs.onlinelibrary.wiley.com/doi/full/10.1002/jgrd.50359
“The absolute surface temperature of the world is likely to be between 13.7 and 14.0°C for the 1961–1990 period and 13.9 and 14.2°C for 1981–2010.The spatial detail reveals that most of this difference comes from Antarctica and to a lesser extent Greenland and the immediate coastal areas around these two landmasses.”
Not even global warming, only antarctic and greenlandic warming.
Yonason,
I think we’re getting close to agreement. Tell me if that’s not the case.
“we are talking about how that heat, once added, becomes distributed through the system.”
The distribution is bottom-up, not top-down.
For example: yes, as molecules descend it will rise in temperature. The maximum temperature depends on the height of the atmosphere and lapse rate, but the height of the atmosphere is a function of how hot the bottom is and the availability of untrapped gases. Hotter temperatures cause more outgassing from surface, which gives gravity work to do in creating a vertical distribution. Gravity alone, and pressure alone can not create an atmosphere and give it a temperature.
So as not to cause confusion, the ideal gas should be stated:
nRT -> PV
Agree?
“Gravity alone, and pressure alone can not create an atmosphere and give it a temperature.”
That’s my understanding. You also need heat added to the system. But, once the atmosphere has heat added to it, if a parcel of that air rises adiabatically it will cool, and if it falls adiabatically it will warm.
Here’s something I posted once to prove that…
See Chapter 8 (P.7). Mine Ventilation Thermodynamics, here…
https://www.mvsengineering.com/files/Subsurface-Book/MVS-SVE_Chapter08.pdf
“This result [equation 8.9] is usually rounded off and quoted as a dry bulb temperature adiabatic lapse rate of 1 °C per 100 m depth. The increase in temperature is a result of potential energy being converted to internal energy as the air falls through the shaft. The effect is also referred to as autocompression.”
That’s why the temperature increases with depth in places like Death Valley and The Grand Canyon.
So, if you are saying that gravity ALONE doesn’t generate PERMANENT heat, then we are probably in agreement, or very close to it.
ASIDE – As the atmosphere warms or cools adiabatically it neither gains nor loses energy, which it seems to me it should if CO2 were absorbing/radiating IR heat in any appreciable amount. But the adiabatic condition holds approximately very well, so it seems to me that the atmosphere cannot be the vehicle for transferring large amounts of radiant energy anywhere. I’m willing to modify that opinion, but only if someone can show me quantitatively how it works.
OOPS
This…
“So, if you are saying that gravity ALONE doesn’t generate PERMANENT heat, then we are probably in agreement, or very close to it.”
Should have been…
“So, if you are saying that gravity ALONE doesn’t generate a PERMANENT temperature profile, then we are probably in agreement, or very close to it.”
Yonason,
Well, yes, heat is a given. That’s what I mean by the Temperature created the atmosphere. The Temperature is set by
1) Insolation
2) Geothermal
3) Stars (Ultra weak)
“The increase in temperature is a result of potential energy being converted to internal energy as the air falls through the shaft. The effect is also referred to as autocompression.”
I have a problem with the causality here. There is “no” increase in temperature as you go down, there is a decrease in temperature as you move away (up) from the geothermal source. Yes, it gets hotter as you move closer to the source, but thermodynamic Causality runs the other way … hot to cold.
Venus is hot due mainly to its geothermal emission, which creates a thick atmosphere with high pressure. The sun alone would only create an atmosphere 10x thinner.
I just wanted to clarify that its not the atmospheric pressure that enhances incoming solar heat.
“I just wanted to clarify that its not the atmospheric pressure that enhances incoming solar heat.” – Zoe Phin
That is correct. But it doesn’t help. While atmospheric pressure doesn’t “enhance incoming solar heat,” it can change the temperature as per T~P*V.
You do realize that heat is extensive and heat is intensive, don’t you? Pressure is also intensive. You can have a wide range of temperatures and pressures for the same quantity of heat.
The atmosphere has weight, and near the surface it is heaviest. The weight of it on itself compresses it, decreasing the volume and causing the temperature to rise, even when the heat content is the same at a higher level with lower pressure and temperature.
The fellow I was quoting is a professor of engineering,…
Malcolm J. McPherson * B.Sc., Ph.D., C.Eng., FIMinE, FIMM, Mem.AIME, Mem.ASHRAE Emeritus Massey Professor of Mining Engineering and former Associate Dean for Research and Graduate Studies, College of Engineering, Virginia Polytechnic Institute and State University. Chairman, Mine Ventilation Services, Incorporated
What I quoted from him is standard compressible fluid dynamics.
I don’t know what else to tell you.
I’m not having a good day proof-reading.
“You do realize that heat is extensive and heat is intensive,…?”
…should have been…
“You do realize that heat is extensive and temperature is intensive,…?”
Yonason,
Good talk. You’re making me more convinced I’m right. I thought about this a lot over the last 3 months.
“While atmospheric pressure doesn’t “enhance incoming solar heat,” it can change the temperature”
No, I don’t think so. I should have said it can’t increase both heat and temperature.
“You can have a wide range of temperatures and pressures for the same quantity of heat.”
Totally agree. However, have you noticed that as you move DOWN the atmosphere, mass and heat capacity AND density increase.
The same amount of heat could create higher temperature, IF you reduce mass/heat capacity.
Here’s a problem:
Assume a 0 kelvin new Venus coming in to the solar system where Venus is.
Assume it has the same thick atmosphere (though one wonders how it could be supported). Question: Can the sun and pressure re-create today’s Venus?
The sun can only deliver 2604 W/m^2 at ZENITH to Venus. Forget even albedo for the moment. What further “temperature increasing” mechanism could there be? The top of the atmosphere can get to 2604 W/m^2 – but this will make those molecules less dense then those below, hence there will be no convection down. You’re only left with conduction. Now, your conduction is climbing down an ascending mass/heat capacity gradient, and therefore temperature decreases.
What happends is that you have an upside-down gradient from 2604 W/m^2 to 0 W/m^2 and not to ~16900 W/m^2 as exists.
“The atmosphere has weight, and near the surface it is heaviest.”
The atmosphere has no weight. It has mass, but no weight. It is countered by a bouyant force caused by temperature. If it had weight, it would fall. Instead it hovers.
“The weight of it on itself compresses it, decreasing the volume and causing the temperature to rise”
There is no net compression. The ideal gas law is:
PV=nRT
Pressure is constant. Volume is constant. A decreasing volume would lower T anyway. But along with increasing pressure, T stays the same.
Again, I think you have it backwards. As I said before:
“yes, as molecules descend they will rise in temperature. The maximum temperature depends on the height of the atmosphere and lapse rate, but the height of the atmosphere is a function of how hot the bottom is and the availability of untrapped gases. Hotter temperatures cause more outgassing from surface, which gives gravity work to do in creating a vertical distribution.”
It is the temperature that sets the pressure, not the other way around.
Yonason,
It takes energy to do work. How much energy does it take to lift gases 10km into the air? (the air of the atmosphere that exists because energy created the “room” and the lift for air to be their)
The atmosphere is a heat sink. Nothing in it (especially GHGs) nor it itself raises surface temperatures. Temperature creates gas pressure via ideal gas law:
nRT -> PV
Once the air is lifted off the ground it gets “compressed” by gravity. Gravity is given work to do. Gravity would like to crush the air back into the ground. This counter force makes some people believe that gravitational pressure is what raises temperature. Whereas in reality, temperature gave gravity work to do compressing the gases it outgassed from the surface.
Basics:
Temperature is average kinetic energy of RANDOM moving particles.
Gravitational pressure is kinetic energy of non-random towards-center-of-mass DIRECTED particles.
I just don’t see how DIRECTED molecules would cause more RANDOM motion – the temperature to go up. I could only see how DIRECTED motion would prevent RANDOM motion from leaving planet entirely (super free expansion).
Boil a closed pot filled with water.
Did temperature create steam and its vapor pressure?
or did steam create the very temperature that boiled it?
Don’t forget:
http://ircamera.as.arizona.edu/NatSci102/NatSci102/text/hydrosta.gif
Atmospheric pressure is in opposite direction to gravity.
Temperature creates pressure.
Some people see the effect and confuse it for the cause.
“Temperature creates gas pressure via ideal gas law:
nRT -> PV”
OK, Zoe, I think I see the problem. The p,v,t equation is deceptively simple. An adiabatic raising or lowering of a parcel of air is a change from say P1,T1,V1 to P2,T2,V2. To calculate that you need to perform an integration, not simple arithmetic. This is the form of the equation we are interested in… PdV+VdP=RdT.
We make our assumptions (for an adiabatic process that’s U=(Q-W)=(0-W)=-W ), set our boundary conditions, rearrange and solve. Here’s a 3-part video, the last part of which discusses adiabatic processes.
https://youtu.be/nkOtOMNS5bQ
(the whole thing is good, because it shows how, by using the same equation we can get different results, depending on the thermodynamic constraints we impose. While pressure and volume will change, those changes do not cancel each other out, as you appear to be assuming. Maybe this will help?
https://www.youtube.com/watch?v=hunmkRGBvBM
Yonason,
It’a not that I don’t understand the adiabatic process. I do. I just don’t think it has anything to do with SURFACE temperatures. I’ll tell you why.
The 1st LoT:
ΔU = Q – W
(You forgot the delta)
The law describes a CHANGE in internal energy. It tells you nothing about the current internal energy at the surface.
In the adiabatic process there is no NET heat flow within the compress gas. It can still have heat entering and leaving. leaving = entering.
The adiabatic process explains the distribution from bottom-to-top, but doesn’t explain the initial conditions.
The distribution in the gas column is irrelevant to surface temperature, but not vice versa.
https://en.m.wikipedia.org/wiki/Polytropic_process
It doesn’t matter which thermal process you choose, C is constant and C=nRT.
It is surface T that sets surface T. This might seem like a stupid tautology, but it explains why Pressure has nothing to do with setting surface temperature.
Joules 2nd law:
“Joule’s second law states that the internal energy of an ideal gas is independent of its volume and pressure, depending only on its temperature.”
Yonason,
The adiabatic process requires initial conditions that have nothing to do with the adiabatic process. The adiabatic process does not create its initial conditions. You can determine what those initial conditions were by reverse engineering the equation, but you can’t say that’s a thermodynamics effect – only the cause.
“(you forgot the delta)”
No. I don’t have it on my keyboard and I was pressed for time. But, if you want to get picky, you forgot it yourself, twice.
“ΔQ=ΔU+ΔW”
https://hs.umt.edu/physics/documents/BOREALIS/Lapse%20Rate%20Terms%20and%20Formulas2012.pdf
Here’s another ref that asserts lapse rate in mine shafts is an important consideration.
https://opendocs.ids.ac.uk/opendocs/bitstream/handle/20.500.12413/4964/roberts,aeimrreportno.163..pdf?sequence=1
“The adiabatic process does not create its initial conditions.”
I never said it did. That’s determined by the boundary conditions you said didn’t exist.
Looking below, I see you don’t like what John Brown writes, either. I’ll make a quick comment there.
Pressed for time all week, so not much more for a while.
Zoe,
you miss the elephant in the room:
you say:
It is the temperature that sets the pressure, not the other way around.
No, it is the mass that sets the pressure through gravity.
The temperature gradient is set by gravity and heat capacity.
John,
“The temperature gradient is set by gravity and heat capacity”
That’s true. The gradient from hot to cold is set by gravity and heat capacity. But the hot surface is not set by those things. The hot surface determines how much atmosphere there is for gravity to compress.
There is NO atmospheric mass, until the temperature and availability of untrapped gas solids/liquids on the surface creates one. If the temperature is too low, there is few gases.
Look at any gas phase diagram. Pressure (mass*gravity) does not create gases. Only temperature does.
Zoe,
We did not talk about where the gas comes from. Since it is there and we discuss pressure, then all I am saying is that the pressure is set by the mass of the atmosphere and gravity.
It almost does not matter at which temperature it is, as long as it is a gas. There is enough phase changes happening to warrant the known pressure changes in the atmopshere, with water being the constituent of interest.
But the main message is, that, and here we have to say, the average, normal standard pressure is set by mass and gravity alone.
John,
That’s wrong.
Atmospheric pressure runs in the opposite direction of gravity.
http://ircamera.as.arizona.edu/NatSci102/NatSci102/text/hydrosta.gif
The gas laws were derived by heating a gas in a container and seeing how the lid/piston moved with varying temperature. The weight of the piston did nothing for the temperature of the gas.
Gravitational pressure wants to crush the gas molelecules, but temperature creates an outward gas pressure that prevents it from doing so.
Mass and gravity do not create its own anti force.
Pressure can’t create gases.
https://upload.wikimedia.org/wikipedia/commons/thumb/0/08/Phase_diagram_of_water.svg/700px-Phase_diagram_of_water.svg.png
Start at the bottom left. Move up the pressure axis. Pressure can’t create an atmosphere. Move right on the temperature axis. Temperature can create an atmosphere that will then be compressed by gravity. Someone then sees this compression and then thinks it was the compression that created the temperature. That’s circular reasoning and confusing effect for the cause – lifting one’s self by their bootstraps.
Zoe,
there is your problem right there:
“heating a gas in a container”
There is no container in the atmosphere.
“Gravitational pressure wants to crush the gas molecules, but temperature creates an outward gas pressure that prevents it from doing so.”
So yes, but this does not set the temperature, but neither does the temperature set the pressure.
The mass does not change with pressure, neither with temperature. The mass and gravitation sets the pressure.
I am not saying that pressure creates gases. If you misconstrue what I am saying we have no discussion.
And as you yourself said,you have to observe the phase changes. Apparently at the atmospheric pressure the matter in the atmosphere is gaseous, particularly CO2, O2 and N2. If you look at the phase diagram of Water, you will have a hard time to even consider water a gas in the atmosphere.
Tell me, if you take the ideal gas law and you increase the pressure by increasing the mass or the gravitational constant. What happens to the temperature side?
Bear in mind that pressure is set alone by these two properties. Temperature will have only an effect on the volume/density in the open system.
Zoe,
also what is wrong with atmospheric pressure runs in the opposite direction of gravity?
Simply higher up there is less mass which the gravity can act on, while also gravity constant slightly gets lower with height.
Nothing wrong with this picture.
@John Brown
I’ve posted some of this guys material before. Here’s something on air pressure.
https://www.youtube.com/watch?v=P3qcAZrNC18
It won’t help Zoe understand why the bases of the Grand Canyon, and Death Valley are always warmer than their rims, due to increased atmospheric pressure there. But it’s fun to watch.
(And, no, Zoe, I’m not saying the pressure alone “causes” the temperature, but that for a fixed energy the lower atmosphere will (usually) be warmer than the upper.
John,
You are so confused.
“Tell me, if you take the ideal gas law and you increase the pressure by increasing the mass or the gravitational constant. What happens to the temperature side?”
How are you going to increase the mass of the atmosphere?
The gravitational constant is irrelevant for the surface temperature.
Yonason,
You too are trying real hard to not understand what I’m saying.
My point is about the temperature on the surface determining the size and pressure of the atmosohere.
Thermodynamic causation runs from hot to cold. Just because you’re physically going from cold to hot doesn’t mean it wad caused in that direction.
Cold + Pressure does not make Hot. You’d have to believe in backconduction and backconvection, which is just as stupid as alarmists’ backradiation.
Gravity’s directed motion does not increase random molecule motion (temperature).
Isn’t what I’m saying obvious? Why are you guys fighting it?
John isn’t confused, Zoe.
“How are you going to increase the mass of the atmosphere?”
Replace a light gas with a heavy one, or vice versa. Most easily accomplished by moving to another planet.
“The gravitational constant is irrelevant for the surface temperature.”
So? Once a parcel of air acquires energy by contact with the ground (convection), it can ascend into the atmosphere and cool, or descend into a valley and warm. And it is the force of gravity that determines the pressure at any height, which determines how warm or cool that parcel becomes.
No more time for a while, and probably shouldn’t be bothering with this. Anyway, see you in a week or two.
Yonason,
“Once a parcel of air acquires energy by contact with the ground.”
Why is that important? Are you saying the maximum T of an air parcel in an atmosphere is determined by energy available at the surface? Hmm…
Yon,
not only heavy gas, just more will do. Increasing the mass is as simple as adding more gas.
Since there is no walls, the atmosphere can grow in height.
The simple answer to the question of the atmospheric pressure is gravity and mass. Adiabatic work in the atmosphere is compression or expansions, both are a measure of the volume.
If one is to take the ideal gas law for the average atmosphere, the pressure is constant, temperature and volume are variable.
Real world observation shows a higher atmosphere over the warmer equator compared to the lower atmosphere at the poles.
Zoe the atmosphere has no walls, so there can be no impact of temperature on pressure by keeping volume constant.
Your point that temperature has any impact on pressure is wrong.
You might be thinking that the pressure can also be calculated by density(profile) and height, where density is affected by temperature. But there is no net effect as temperature changes height and density at the same time through expansion and compression with the impact on volume. Cold air more dense and takes less volume, the cold air column will be less high.
With more mass it will be higher. So mass is the determining factor for the pressure.
John,
“Increasing the mass is as simple as adding more gas.”
LOL. And what mechanism brings more gas into the atmosphere?
“the atmosphere can grow in height.”
LOL. By what mechanism? Gravity and existing atmo mass, or by outgassing due to higher temperatures? Where does the energy come from?
“Adiabatic work in the atmosphere is compression or expansions”
LOL. Gravity can only compress …
The atmosphere is not purely adiabatic. It’s also diabatic.
“Since there is no walls, the atmosphere can grow in height.”
The height is determined by temperature. For gas molecule to rise to height h it needs mgh energy. Where do you think it comes from? It doesn’t come from the atmosphere itself, genius.
I don’t understand why people are trying to put the cart before the horse.
The atmosphere is a heat SINK, not an engine.
Zoe,
your discussion style deteriorates.
If you have can prove that temperature has a discernible effect on the average pressure in the atmosphere prove it.
Did we talk about energy? At least I have not. Not sure why you suggest the things you do.
I know the atmosphere is a heat sink for the surface. There we agree.
What sets the pressure is the question, and surly temperature is not.
John,
Why do you deny Gay-Lussac’s Law?
Zoe,
do I?
I deny that you can claim that the atmosphere has a constant volume.
You are applying the wrong law to the question at hand and yet you also missing the point that the amount of mass is not affected by temperature.
John,
Can you find any gas law that claims that pressure controls temperature? Go ahead. How could it do so without effecting volume? And any such process would be temporary anyway.
Ofcourse the amount of mass in the atmosphere is controlled by tempetature. What is the atmosphere but gas molecules moving in random directions?
How would gravity, a directed force, cause random motion? It can’t. It can only contain atmo mass determined by temperature.
Zoe,
I can find an application that proves it. The compression cycle in a combustion engine for example.
The ideal gas law would be a law to show an effect of pressure onto temperature.
It seems that Gay-Lussac’s Law as a deviation from the ideal gas law (constant volume) suggests the same.
The mass in the atmosphere is not controlled by temperature. State the pertinent law please if you want to continue this discussion.
I never claimed that gravity is causing random motion. I said that gravity through mass creates the pressure.
Do you agree that the volume of the atmosphere is variable?
@John Brown 30. October 2019 at 6:29 PM |
“not only heavy gas, just more will do. Increasing the mass is as simple as adding more gas.”
Of course.
“Zoe, your discussion style deteriorates.”
Her material consists of some errors interspersed with correct statements, and she’s constantly shuffling that deck. Seems to enjoy arguing.
Just an observation on her last comment…
_________________________
“Can you find any gas law that claims that pressure controls temperature? Go ahead. How could it do so without effecting volume?”
Paraphrasing, ‘pressure doesn’t control temperature, except when it does.’
“And any such process would be temporary anyway.”
…which is why we have weather. (And she tells me I’m making things too complicated?)
_________________________
Just one more comment on one error she makes.
“‘the atmosphere can grow in height’. (John)
LOL. By what mechanism?” (Zoe)
Like THIS, Zoe.
What are the bets she still accuses me of being wrong because the cold poles have higher pressure than the warm tropics, and doesn’t even mention the fact that she has been shown incorrect.
Yonason,
There is a lot of sophistry in your comment.
Sorry but clouds don’t raise the atmosphere. They exist within the atmosphere.
The poles also have higher density gases. It takes more energy to push them up. And see, even you yourself agree yhat greater pressure doesn’t make it warmer. QED
I asked for a reference. Why can’t you give me one? Because all respectable references will say temperature controls pressure, and not vice versa.
Please stop trying to win an argument, and learn something. I would have wholeheartedly agreed with the adiabatic-compression-enhances-warmth theory a few months back, but I see it’s just not so. It reverses causality.
BTW, I started a blog.
http://phzoe.wordpress.com
Would appreciate some comments there.
Thank you for your efforts. Peace and take care.
“Sorry but clouds don’t raise the atmosphere.” – Zoe
But the height of the atmosphere determines how high the clouds can rise, as that link explains.
“The troposphere has different depths at different places around the Earth. The troposphere is deeper, or higher, near the equator, and it is thinner near the poles. This is because the air is warmer near the equator than at the poles. The Sun heats the Earth mostly at and near the equator, and this warm air rises and causes the troposphere to be deeper above this part of the planet. At and near the poles, the air is cooler and sinks, so the troposphere is thinner above this part of the planet. Because of this, high clouds in the tropics have a higher base and a higher top than high clouds in the mid-latitudes or high clouds in the tropics.“
I.e., the cloud height is linked to the height of the atmosphere, which IS different at the poles than at the equator.
Come on, Zoe, please read the WHOLE thing. As you write, “Please stop trying to win an argument, and learn something.” //:o]
@Zoe
I don’t want it to seem as if I think you are wrong about everything. I don’t. You get a lot correct. And the few things you do seem to have wrong should be easy to fix.
Good luck with your blog.
Took a quick look at your blog. No time to read and comment now, but will in a few days. It looks promising.
Here’s some material you may be interested in, based on what I saw there.
https://rclutz.wordpress.com/2019/09/14/global-warming-theory-and-the-tests-it-fails/
https://youtu.be/XfRBr7PEawY
I’m posting them here as opposed to your blog, because I think other NTZ readers will be interested.
Yonason,
You’re a funny guy 🙂
“But the height of the atmosphere determines how high the clouds can rise, as that link explains.”
I don’t disagree with the link, but the question was what determines atmo height.
Thank you for nice comments.
Yonason,
What is the air temperature at 2 bars of pressure here on Earth?
Check out this side of thermodynamics:
https://www.mvsengineering.com/files/Subsurface-Book/MVS-SVE_Chapter08.pdf
For your thought experiment of the 2 bars you need to add enough atmospheric mass to answer the question.
LOL. You need to raise surface temperature so that the atmosphere grows by outgassing enougg gases to raise pressure to 2 bars. But the surface doesn’t receive that much energy. Throwing gases up in the air will not keep them there.
Zoe, is that so?
Are you saying the mass of the atmosphere is controlled by temperature?
Last I looked the triple point of Nitrogen suggests otherwise.
Also last I looked a gas remained a gas thrown or not.
Its kind of an oxymoron to thrown gas in the “air” really.
John,
If a planet is at 0 kelvin, there will be no outgassing at all, and no atmosphere at all. Atmospheric mass and pressure would be zero.
On this planet, if you dropped an anvil, the gases would briefly rise up and fall. The anvil now exerts a pressure on the surface, but the temperature settles back to 0K.
Zoe,
Matter will always have a temperature but this would be a more theoretical discussion.
You correctly pointing out the obvious, that something that isn’t there , does not have properties. Why?
And why did the anvil fall?
Again it is a theoretical discussion, which we will not have.
Enjoy the weekend.
“In the adiabatic process there is no NET heat flow within the compress gas. It can still have heat entering and leaving. leaving = entering.”
Oops, I meant to say:
There is no heat exchange with surroundings. Q=0. All the surface energy will be used to do work, i.e lift the gas molecules and create a pressured gas volume. The surface will be dissipating energy doing so – reduce its temperature. That’s why the atmosphere is a “heat” sink.
The surface must have enough energy not only for its own Temperature, but lift the gas molecules.
The lifted gas molecules that are noe being “compressed” by gravity do not add to the energy of the surface to raise its temperature. Such a thing would just undo what was done.