According to McElhany, 2017, “there are no studies that directly demonstrate modern day effects of OA [ocean acidification] on marine species.” There still aren’t.
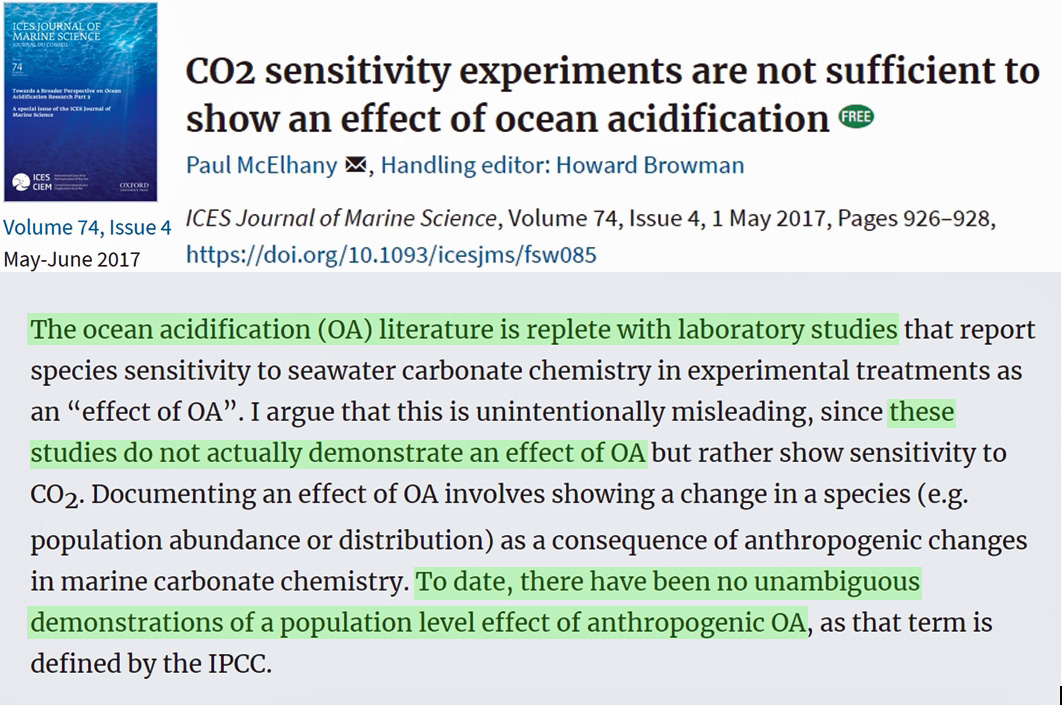
Image Source: McElhany, 2017
Scientists claim that the ocean’s global mean surface pH may have declined (i.e., became less alkaline and thus more “acidic”) by -0.08 in the last 265 years — from 8.13 during pre-industrial times to 8.05 today. That’s an overall, long-term pH change rate of -0.0003 per year.
By way of comparison, from one season to the next, or over the course of less than a year, pH levels naturally change by twice that amount (±0.15 pH units). On a per-decade scale, oceanic pH can naturally fluctuate up and down by up to 0.6 units within a span of a decade (as shown in red below).
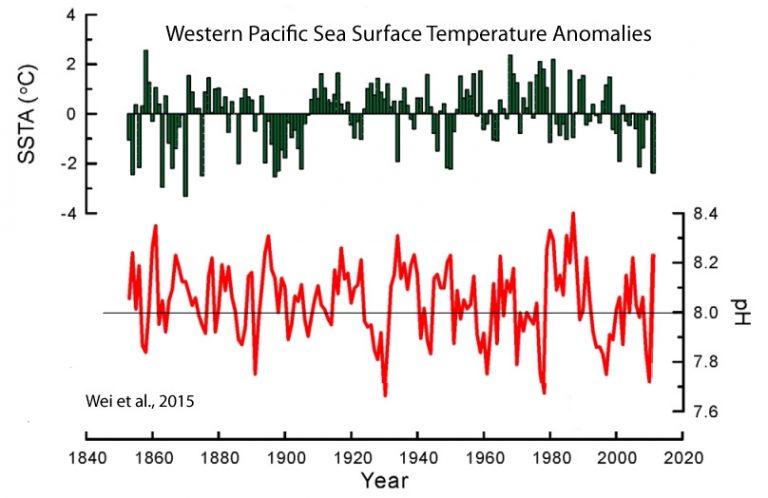
Image Source: Wei et al., 2015
Without valid scientific explanation or detailing the driving mechanism, prognosticators of CO2-induced climate doom are claiming (via modeling) that the -0.08 change in 265 years will morph into a -0.4 change in the next 82 years!
Even worse, experiments have revealed that marine species (i.e., corals) naturally survive in the “acidic” environments predicted by models, thereby demonstrating that we still have no scientific confirmation that we humans are actually harming the oceanic biosphere with our CO2 emissions.
“Uptake of anthropogenic CO2 is changing the chemistry of the global ocean [1]. When entering the sea, CO2 reacts with water to form carbonic acid, and this ocean acidification (OA) has lowered the global ocean mean surface pH from 8.13 during the pre-industrial age to the present day 8.05. This trend is predicted to continue and current models estimate a further decrease of up to 0.4 pH units by the year 2100.”
“We incubated females at present day conditions (pHT 8.0) and year 2100 extreme conditions (pHT 7.5) during oogenesis and subsequently reciprocally transplanted laid eggs between these two conditions. Statistical tests showed no effects of maternal or direct exposure to OA [ocean acidification] at this level [7.5 pHT].”
“For example, over seasonal time-scales Porites corals from the Great Barrier Reef (GBR) have a large range in pHcf of ~8.3 to ~8.5, significantly greater (~×2 to ~×3) than that of reef-water (pHT ~8.01 to ~8.08), and an order of magnitude greater than that expected from ‘static’ laboratory experiments.”
“Strong physiological controls, but of a different character, are found in corals grown in a Free Ocean Carbon Enrichment Experiment (FOCE) conducted in situ within the Heron Island lagoon (GBR). These corals exhibit near constant pHcf values regardless of external changes in temperature and seawater pH. This pattern of strong physiologically controlled ‘pH-homeostasis’, with elevated but constant pHcf has been found despite large natural seasonal variations in the pH (±0.15 pH units) of the lagoon waters, as well as the even larger super-imposed decreases in seawater pH (~0.25 pH units) designed to simulate year 2100 conditions.”
“In natural reef environments we thus find that the processes influencing the up-regulation of pHcf in symbiont-bearing corals are subject to strong physiological controls, behaviour that is not well simulated in the current generation of aquaria-based experiments with fixed seawater pH and temperature.”
“Over a period of 32 days, larval survival, growth in size and weight, and instantaneous growth rate were assessed in a crossed experimental design of two temperatures (10°C and 12°C) with two CO2 levels (400 μatm and 900 μatm CO2) at food levels mimicking natural levels using natural prey. Elevated temperature alone led to increased swimming activity, as well as decreased survival and instantaneous growth rate (Gi). The comparatively high sensitivity to elevated temperature in this study may have been influenced by low food levels offered to the larvae.”
“Larval size, Gi and swimming activity were not affected by CO2, indicating tolerance of this species to projected “end of the century” CO2 levels. A synergistic effect of elevated temperature and CO2 was found for larval weight, where no effect of elevated CO2 concentrations was detected in the 12°C treatment, but a negative CO2 effect was found in the 10°C treatment. Contrasting CO2 effects were found for survival between the two temperatures. Under ambient CO2 conditions survival was increased at 12°C compared to 10°C.” [Survival rates increased with warmer water temperatures]





The Canadian Marine authorities state the Ph of the seas are stable between 7.5-8.5.
Marine authorities around the world state safety levels for marine life at between 6.5 – 9.5
The way I read a pH chart it seems to me the way to say it is that it is less alkaline or basic and thus closer to neutral. How can something be more acidic when it has a pH number higher than neutral? Acidic does not start until you go lower than 7 or neutral.
Some may think I’m splitting hairs but then again that is part of what science is about isn’t it?
I’m just always sensitive to the language that is used and the premise presented because people have constantly been misled using such tactics.
“Acidic” sounds worse than “less alkaline”.
Similarly, saying that “Greenland has lost a staggering 9,000 gigatons of ice in the last 110 years” sounds worse than saying “Greenland’s ice melt has contributed a grand total of 1 inch to sea level rise in the last 110 years”. Hence the reason why the headlines read the way they do…
https://notrickszone.com/2018/02/12/the-epic-failure-of-glacier-melt-sea-level-rise-alarmism-continues-to-bespoil-climate-science/
Greenland total ice mass since 1900.
https://s19.postimg.cc/9i1vx9lv7/Greenland_ice_mass2.png
SCARY LOSSES !! 😉
RAH, you’re right of course, but the language chosen is just one more proof of a conscious, paid-for hoax.
Joe Public, as Kenneth said, thinks “more acidic” is scarier than “less alkaline”. Actually, I do believe that alkaline is scarier to the fish, and requires them to defend against it.
Most human food, including fish, meat, vegetables, grain, etc is acid, as in amino acid.
And you wonder why you guys are called conspiracy theorists? 😉
Well, there are usually only two directions on such a scale. Accelerating/Decelerating, louder/quieter, warming/cooling, acidification/alkalinisation, etc … would you say that is not cooling, but less warming happens if the temperature drops? Weird.
It’s not about fish at all, it’s about the much smaller life at the beginning of the food chain.
” it’s about the much smaller life at the beginning of the food chain.”
Don’t worry seb.. you and your AGW scum will survive.
You actually NEED CO2.. you just are too brain-hosed to admit it.
Well, there are usually only two directions on such a scale.
No, nobody is wondering that. We know you have little to offer other than name-calling.
“you guys are called conspiracy theorists”
ROFLMAO.
Again, you are the one bringing up the word.
I’ve never been called that by anyone except you.
And from you it is MEANINGLESS.
Don’t you have enough mirrors at home, seb ???
Even your own priests have said what the REAL purpose behind the Anti-CO2 AGW-cult agenda is.
The term acidification of the seas was coined by Caldeira back in 2003 to frighten the witless.
Behind the scenes this is what he wrote-
“This is often termed “ocean acidification” because it describes the
process of decreasing pH. Current projections of ocean acidification
suggest that
the pH of surface ocean waters will continue to decline. However, the
term can also lead to confusion
when it is wrongly assumed that the oceans will become acidic, when in
reality, ocean pH is never expected to fall below 7.0; i.e., the
oceans are becoming
less basic, but not acidic. Such a phenomenon could
only occur in the unlikely event that CO2 emissions
reach more than 10,000 Pg C (Caldeira and Wickett,
2005)
Spot on, but he still kept on publicly pushing the scary theory. Caldeira featured in a film by the National Resources Defense Council in August 2009, called “Acid Test: The Global Challenge of Ocean Acidification”, narrated by Sigourney Weaver. He participated in several Royal Society reports on “Ocean acidification due to increasing atmospheric carbon dioxide”
More background on this here:
“Acid Seas – Back To Basic”
http://scienceandpublicpolicy.org/science-papers/originals/acid-seas
On the pH scale whenever one pH is 8 or 5->4. in the first case you are right that they are both alkaline but the 8 is “more” acidic than 9 by quite a bit. Remember that pH is a logarithmic scale.
no, 8 is not “acidic” at all..
so it can’t be “more acidic”
Yes, it has a greater concentration of H+ ions, but it is not acidic, any more than caustic soda is acidic.
to use a sebian analogy..
if my bank account goes from negative $2000 to negative $1000, do I have more money in the bank ?
The very concept of pH became current in the early 1900’s. The first commercial pH meter was released in 1939.
Yet these people claim to know the pH of sea water for the last 265 years. How do they know it has declined? By assuming that it has declined and making up a figure (sorry! using a computer model) that confirms their assumption. This is
Chemically
Related
Alarmist
Propaganda.
Graeme, how do we know the temperature from 265 years ago? How do we know it from 1000 years ago? How do we know the TSI from before the satellite age?
Are reconstructions a new concept to you?
Temperatures can be reconstructed by the O18/O16 ratio, well known and fairly accurate science. How do we know the TSI from before the satellite age – we don’t because you and you fellow believers claim that the sun has never varied, so you can claim that the (adjusted) recent figures are something “unprecedented” ( to use a much overworked, indeed abused word). Others use the C14 level or the Be10 level as a measure of solar strength.
But how do you reconstruct a pH? With a nonexistent pH meter?
Clear off, SebastianH, you know nothing about Science only Dogma from the Church of Self Deluding believers in a Flat and never varying Earth.
You should have tried googling “pH reconstruction” as well …
Only the VERY deluded think they can “reconstruct” ocean pH from before the industrial revolution to 0.1pH accuracy.
Oh.. its seb..
Since the authors of the original paper didn’t mention diatoms so bloody what? They weren’t in a lake with big pH changes, they just ASSUMED that the pH was affected by the level of CO2 and extrapolated back to some impressive sounding date and manufactured a pH figure. They then used this as conformation of their claim.
Obviously SebastianH you aren’t a scientist and not very strong on logic either.
Reconstruction of molecular concentrations are feasible. Reconstruction of H503 (hydronium ion) or OH ion (pH) is little more than wishful thinking. Exposure to the ice lattice, CO2. CO3, O2, or any other lewis acid or base ( all those salts in sea water) would change the ph in a variety of nonlinear ways. this could not be reliable modeled, though that would stop anyone
All historic measurements of ocean surface pH seb
https://s19.postimg.cc/ewnhcf8bn/ocean_PH_all_surface_readings.png
Purely alkaline and purely staying that way. Maybe a slight trend toward more caustic.? what do you see in that REAL DATA, seb?
One day you might actually learn enough chemistry to understand the difference between acidic and alkaline.
But like proof of CO2 warming.. we expect to be waiting a LONG, LONG time for anything but EMPTY mindless rhetorical AGW regurgitation from you.
One day you might learn to comprehend words …
But perhaps you do say your tea has become less warm instead of “it cooled down”. Anyway, calling a decrease on the pH scale acidification is perfectly fine.
Real data: https://www.pmel.noaa.gov/co2/file/Hawaii+Carbon+Dioxide+Time-Series
https://www.pmel.noaa.gov/co2/story/Ocean+Acidification
roflmao
Another FAILED seb analogy, showing he has zero competence in chemistry.
Aloha was just part of the normal cycle of pH swings
https://s19.postimg.cc/3tmdoeac3/p_Hand_CO2.png
Were you UNAWARE of that , seb ? (add it to the LONGGGGG list !!)
And surly you aren’t dumb enough to put any importance in such a short term study when there are KNOWN CYCLES.
And a link to a low-science NOAA propaganda page intended just for gullible, low-information people like you.
*SIGH !!!*
” calling a decrease on the pH scale acidification is perfectly fine.”
Iff you are doing it for propaganda purposes.
Scientifically .. Not so much.
But you stick with the propaganda, seb
Science seems to be BEYOND you.
The correct term when you lower a pH .7 using an acid reaction is neutralisation.
And there is ZERO evidence except from assumption driven models that the oceans are neutralising. They remain steadfastly around pH 8.1 even with all the rivers , that are often acidic, pouring into them over millions of years.
No feeble change in a minor trace gas is EVER going to change that pH in any way what so ever.
Its just ANOTHER hoax from the CO2 HATING AGW-cultists.
Hmm, are you “VERY deluded”?
“when you lower a pH .7”
OOPS a “greater than ” sign doesn’t work, does it,
read it as
“when you lower a pH greater than 7…using an acid reaction, it is called neutralising ” NOT acidifying.
chemistry 101, for seb, when he gets that far.
“are you “VERY deluded”?”
You are the one that “believes” in a change of 0.1 pH.. ZERO REAL EVIDENCE
You are the one “believes” CO2 causes warming.. ZERO EVIDENCE
Basically everything you “believe” about climate has ZERO-EVIDENCE.
That makes you the DELUSIONAL one.
Either that or mind-numbingly GULLIBLE.
We have tree stumps in the subarctic and Arctic from 1,000 years ago that are in areas covered in permafrost or ice today. We have land-based water level markers that clearly show how long ago sea levels were meters higher than they are now. Proxy records from tree rings, fossils, etc., also provide evidence that it was (much) warmer 1,000 years ago.
The graph of all ocean surface pH readings over time looks like this.
https://s19.postimg.cc/ewnhcf8bn/ocean_PH_all_surface_readings.png
But…but– colder water holds more CO2, so are they saying it’s colder now than 265 years ago?
Or do warmists not admit that colder water holds more CO2 & is therefore less alkaline?
I’m getting confused. Isn’t the sea already saturated w CO2, so that human’s can’t add to it? Water holds a certain fraction of CO2 depending on the temperature, no? I seem to remember something called Benny’s Law.
Can anyone straighten me out on this? I’m sure the warmists can’t have made such an obvious error, so it must be me.
http://www.principia-scientific.org/ocean-acidification-claims-are-misleading-and-deliberately-so.html
“Either the oceans are getting warmer and the CO2 concentration in seawater is decreasing, which means that ocean acidification from man-made CO2 from the atmosphere is nonsense. Or the oceans are getting cooler and the man-made CO2 from the atmosphere is dissolving in those cooler oceans and causing – insignificant – ocean acidification, which means that warming oceans and the associated sea level rises are nonsense.”
Scientists acknowledge that “critical mysteries remain” about the ocean as a net carbon sink, that we have an inability to quantify the “mean CO2 sink” observationally (all we have are models) and thus we do not have a “mechanistic understanding of how the ocean carbon sink works”. Furthermore, scientists admit that “it is not yet possible to directly confirm from surface observations that long-term growth in the oceanic sink is occurring” and that this is “sufficient to prevent detection of anthropogenic trends in ocean carbon uptake on decadal timescales“.
—
McKinley et al., 2017
http://www.annualreviews.org/doi/10.1146/annurev-marine-010816-060529
“That the growth of the partial pressure of CO2 gas in the atmosphere ( pCO2 atm) drives a growing oceanic sink is consistent with our basic understanding that, as the globally averaged atmosphere-to-ocean pCO2 gradient increases, carbon accumulation in the ocean will occur at an increasing rate (Section 3). This behavior has been illustrated clearly with models forced with only historically observed increases in pCO2 atm and no climate variability or change (Graven et al. 2012, Ciais et al. 2013). Nonetheless, critical mysteries remain and weigh heavily on our ability to quantify relationships between the perturbed global carbon cycle and climate change.”
“The current inability to accurately quantify the mean CO2 sink regionally or locally also suggests that present-day observational constraints are inadequate to support a detailed, quantitative, and mechanistic understanding of how the ocean carbon sink works and how it is responding to intensifying climate change.”
“This lack of mechanistic understanding implies that our ability to model (Roy et al. 2011, Ciais et al. 2013, Frolicher et al. 2015, Randerson et al. ¨ 2015), and thus to project the future ocean carbon sink, including feedbacks caused by warming and other climate change, is seriously limited.”
“The sum of the available evidence indicates that variability in the ocean carbon sink is significant and is driven primarily by physical processes of upwelling, convection, and advection. Despite evidence for a growing sink when globally integrated (Khatiwala et al. 2009, 2013; Ciais et al. 2013; DeVries 2014), this variability, combined with sparse sampling, means that it is not yet possible to directly confirm from surface observations that long-term growth in the oceanic sink is occurring. … [T]his CESM-LE analysis further illustrates that variability in CO2 flux is large and sufficient to prevent detection of anthropogenic trends in ocean carbon uptake on decadal timescales.”
I find it interesting that Kenneth goes full “I have no clue” here while at other times displaying at least some knowledge about how the exchange of gases between two reservoirs works.
You guys seem to miss the slightly important part about differential pressure that determines how much gets absorbed/released.
Or in other words, if there is no CO2 in the atmosphere it can obviously not be absorbed by the ocean, no matter how cold the water becomes. If there is a lot of CO2 in the atmosphere even much hotter water can still absorb it if the partial pressure of CO2 in the water is less than that of the atmospheric CO2.
Since we are artificially increasing the partial pressure of CO2 in the atmosphere, that additional CO2 is being absorbed by the oceans (about half of it). More CO2 in the oceans means the pH value drops.
Another EMPTY zero-knowledge post from seb.
We are NOT artificially increasing anything.
Coal is a purely NATURAL product. We are putting the lost carbon back into the carbon cycle WHERE IT BELONGS.
And ocean life LUVS CO2, and like the land biosphere has been suffering from a lack of it for many hundreds of thousands of years.
It NEEDS CO2.. what don’t you comprehend, seb???????
Why do you HATE life so much, seb.??
If there is a lot of CO2 in the atmosphere “
There is NOT a lot of CO2 in the atmosphere. It is pretty much at a long term historic low, marginally above plant no-grow levels.
WHY do you continue to show your ABJECT IGNORANCE about basically everything to do with biology, chemistry, science and physics ??
Maybe one day you may actually get around to displaying at least some knowledge..
… but we do not have any expectations.
Clown. Seriously, what are you trying to accomplish with these replies? You are only demonstrating how weird and disinformed skeptics (mostly) are.
Poor seb,
You just met our VERY LOW expectations of you, YET AGAIN
EMPTY, zero-science bluster.
As expected..
the seb way !!
It should be noted that seb was totally unable to counter even one of the facts put forward.
All he could manage was a pitiful, mindless yapping noise.
“Coal is a purely NATURAL product.”
“We are putting the lost carbon back into the carbon cycle WHERE IT BELONGS”
“And ocean life LUVS CO2,”
“and like the land biosphere has been suffering from a lack of it for many hundreds of thousands of years.”
“It NEEDS CO2.. ”
“There is NOT a lot of CO2 in the atmosphere. It is pretty much at a long term historic low”
Each statement true and correct..
No wonder all he can do is yap. !
“Clown. Seriously, what are you trying to accomplish with these replies? You are only demonstrating how weird and disinformed skeptics (mostly) are.”
No seb you are wearing that hat and make-up!
YOU ARE THE DISINFORMER HERE!
You ‘knowledge’ of physics and chemistry is woeful, you comments trade solely on alarmist propaganda and little else. You appear to be recite memes well beyond your capacity to understand their failings.
In less friendly places I would call you a knob.
Indeed Kenneth, Henry’s Law rules, eh?
Ah, thank you. No wonder I couldn’t find it under Benny’s Law.
Henry’s Law: “At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.”
Seb wants to read this as meaning that humans increase the pressure of CO2, since we slightly increase its concentration in the atmosphere.
My rebuttal is that CO2 concentration isn’t CO2 pressure because atmospheric pressure is constant and CO2 regardless of source is a component of that atmosphere.
But what does “PARTIAL pressure of that gas” mean, in the Henry’s Law definition above?
Please keep posting here, Penelope. Your contributions are quite good. And we need a little more balance with regard to the gender of our contributors (if I may make this assumption based upon your name).
This is just awful and Kenneth calls it “quite good” … how fitting :/
You can google what terms like “partial pressure” mean, Penelope (and Kenneth). It’s the pressure one gas of a mixture would have if it occupied the complete volume of the mixture. Thus an increase of the CO2 concentration increases the partial pressure of that gas in the atmosphere.
But please keep posting, at least you are asking questions when you don’t seem to know something. Other skeptics just invent something that sooths their minds when they reach the edge of their knowledge …
So why do scientists find that oceans are net source of CO2?
Reimer et al., 2013
https://agupubs.onlinelibrary.wiley.com/doi/abs/10.1002/jgrc.20319
“The study of air-sea CO2 fluxes (FCO2) in the coastal region is needed to better understand the processes which influence the direction and magnitude of FCO2 and to constrain the global carbon budget. The near-shore region was a weak annual net source of CO2 to the atmosphere (0.043 mol CO2 m-2 y-1); where 91% of the outgassed FCO2 was contributed during the upwelling season.”
—
Astor et al., 2013
http://reef01.marine.usf.edu/sites/default/files/project/cariaco/publications/Astor_et_al_2013.pdf
“Based on these observations, 72% of the increase in fCO2sea in Cariaco Basin between 1996 and 2008 can be attributed to an increasing temperature trend of surface waters, making this the primary factor controlling fugacity at this location. … An increase/decrease of 1°C is usually followed by an increase/decrease of 16–20 matm of fCO2sea. Thus, the SST increase of 1.3°C between 1996 and 2008 accounted for 16 matm increase in fCO2sea explaining around 72% of the fCO2sea observed variation. This suggests that the changes measured in fCO2sea were primarily the result of surface-ocean warming in Cariaco Basin. … These observations confirm that this area is a consistent source of CO2 to the atmosphere. The main process controlling the long-term changes in surface fCO2sea at CARIACO was temperature, with net community production playing a secondary role. … At the CARIACO site, the ocean is primarily a source of CO2 to the atmosphere, except during strong upwelling events.”
—
Ikawa et al., 2013
https://www.biogeosciences.net/10/4419/2013/bg-10-4419-2013.html
“We estimated that the coastal area off Bodega Bay was likely an overall source of CO2 to the atmosphere based on the following conclusions: (1) the overall CO2 flux estimated from both eddy covariance and pCO2 measurements showed a source of CO2; (2) although the relaxation period during the 2008 measurements were favorable to CO2 uptake, CO2 flux during this period was still a slight source; (3) salinity and SST were found to be good predictors of the CO2 flux for both eddy covariance and pCO2 measurements, and 99% of the historical SST and salinity data available between 1988 and 2011 fell within the range of our observations in May–June 2007, August–September 2008 and November 2010–July~2011, which indicates that our data set was representative of the annual variations in the sea state. Based on the developed relationship between pCO2, SST and salinity, the study area between 1988 and 2011 was estimated to be an annual source of CO2 of ~ 35 mol C m−2 yr−1. The peak monthly CO2 flux of ~ 7 mol C m−2 month−1 accounted for almost 30% of the dissolved inorganic carbon in the surface mixed layer.”
—
Rutherford et al., 2016
http://adsabs.harvard.edu/abs/2016AGUOSAH24A0042R
“Continental shelves account for a large proportion of global primary production, and potentially a disproportionate fraction of the carbon dioxide (CO2) flux between atmosphere and ocean. The continental shelf pump hypothesis proposes that continental shelves at high latitudes act as net sinks of atmospheric CO2. However, direct measurements on the Scotian Shelf, off eastern Canada, indicate that this shelf region acts as a net source of CO2 to the atmosphere.”
—
Brown et al., 2015
https://pdfs.semanticscholar.org/33c1/d089bfff02f68500a5270e2461fb224e6656.pdf
“Complex oceanic circulation and air–sea interaction make the eastern tropical Pacific Ocean (ETPO) a highly variable source of CO2 to the atmosphere. … Inter-annual variability was observed within the region, with the location of the western extent of the freshpool moving westwards considerably between 2010 and 2014. Previous work within this region suggest that changes in thermocline depth related to ENSO are likely to influence pCO2 within this region. The region is a net contributor to atmospheric CO2, with average sea to air fluxes (over the four years of observations) of 1.6 mmolm−2d−1, with all regions of the ETPO outgassing year-round, except the rainfall diluted Gulf of Panama/Freshpool region.”
Like the oceans, wetlands and rivers are also a net source of CO2.
—
Biswas et al., 2018
https://www.researchgate.net/profile/Protusha_Biswas/publication/323695965_Urban_Wetlands_-_CO2_sink_or_source_A_case_study_on_the_aquaculture_ponds_of_East_Kolkata_Wetlands/links/5aa64f7aaca272d448baded8/Urban-Wetlands-CO2-sink-or-source-A-case-study-on-the-aquaculture-ponds-of-East-Kolkata-Wetlands.pdf
“[U]nder several conditions, natural ecosystems can be equally responsible for CO2 emission like any other anthropogenic activities which continuously release heat-trapping gases in the process of development. … The present study aims to see whether the aquaculture ponds of EKW complex are acting as a source or a sink. Airwater carbon dioxide (CO2) flux was estimated for three consecutive seasons in a year and it was found that the system is acting as a CO2 source in all the three seasons.”
—
Wang et al., 2018
https://aslopubs.onlinelibrary.wiley.com/doi/full/10.1002/lno.10614
“We conducted a free‐water mass balance‐based study to address the rate of metabolism and net carbon exchange for the tidal wetland and estuarine portion of the coastal ocean and the uncertainties associated with this approach were assessed. … We observed that the overall estuarine system was a net source of CO2 to the atmosphere and coastal ocean and a net sink for oceanic and atmospheric O2.”
—
Li et al., 2018
https://www.sciencedirect.com/science/article/pii/S0022169418300660
“Our calculated CO2 areal fluxes were in the upper-level magnitude of published data, demonstrating the importance of mountainous rivers and streams as a global greenhouse gas source, and urgency for more detailed studies on CO2 degassing, to address a global data gap for these environments. … Rivers have been widely reported to be supersaturated in carbon dioxide (CO2) with respect to the atmosphere, and are a net source of atmospheric CO2 (Butman and Raymond, 2011; Raymond et al., 2013).”
“Other skeptics just invent something that sooths their minds when they reach the edge of their knowledge …”
And seb does it ALL the time, because he is ALWAYS beyond the limit of his knowledge.
seb, the AGW FANTASY CHILD.
His knowledge is SO LIMITED he can’t even support the very basis of the AGW farce.. ie CO2 warming of anything (it doesn’t)
… or CO2 causing oceans to neutralise. (they aren’t)
… So he just invents FANTASY after FANTASY in a clown-like attempt to cover for his ineptitude.
Because it is in many locations? How do you think the (complete and global) ocean as a net sink works? Do you think it uniformly absorbs CO2 evenly distributed over time and space?
Another “ocean as CO2 source” finding
https://www.sciencedirect.com/science/article/pii/S0924796312001674
It is noted that seb YET AGAIN has NOTHING but mindless bluster.
“How do you think the (complete and global) ocean as a net sink works? “
by seb-magination !
That is the ONLY way anything on seb-planet works.
— pure FANTASY, and hallucinogenic imaginings.
And one of my favorites that has never been questioned in accuracy or methodology is From Nature Geoscience 16 OCTOBER 2011 | DOI: 10.1038/NGEO1294.
Significant efflux of carbon dioxide from streams and rivers in the United States.
Also there is AGU RESEARCH LETTER 10.1002/2015GL064222, Hidden carbon sink beneath desert
Yan Li1, Yu-Gang Wang, R. A. Houghton, and Li-Song Tang
Where they say
So if you think you know where all the atmospheric CO2 is coming from and going to, seb, think again.
The jury this side of the argument is still out, and all you are offering a level of certainty that is wholly spurious.
Henrys law can not describe the entire process of the exchange of CO2 with the atmosphere.
Henrys law only applies to gases that do not react with the water like Oxygen and Nitrogen. CO2 on the other hand forms a mild acid as we know and therefore adds another process of the solubility in water.
Looking at the numbers of how soluble CO2 is compared with other gases you will see it is not much that stays gaseous in the water.
Reason being the chemical reaction to form carbonic acid.
Not only that, but there are a myriad of other minerals and compounds involved.
Anyone that uses pure water and only CO2 in their attempted propaganda science equations, (as seb’s NOAA link did). is either having themselves on… or talking just to the GULLIBLE. !
John Brown 26. May 2018
CO2 dissolves in water (it does I’ve done the experiment many times!), only a very small percentage becomes carbonic acid at the usual range of temperatures (less than 1% of DISSOLVED CO2 GAS)
Yep, and the rest becomes what? And what is the pH scale a measure of? Now you know why CO2 being absorbed by the oceans decreases the pH value.
@John Brown 26. May 2018,
Carbonic acid is a minor part of the CO2 dissolved in pure water (less than 1% at normal sea level tempertures and pressures). Over 99% of CO2 remains dissolved in water. Do the experiment and see for yourself. If it did not work this way your soda pop, beer and carbonated water would ‘fizz’ at a very much slower rate!
As a first approximation Henry’s law work for CO2!
See http://ion.chem.usu.edu/~sbialkow/Classes/3600/Overheads/Carbonate/CO2.html for the theory of pure CO2 DISSOLVING in pure water.
Carbonic acid is only a very weak acid, and in seawater, there many other biological processes and dissolved minerals at work. Overall with interactions and reaction by the marine biosphere and the minerals in seawater, such a weak natural acid is very, very unlikely to ever endanger life. Historically elevated atmospheric CO2 and dissolved seawater CO2 have not been problematic for life on this planet, even when CO2 was twice the level of today.
As I know seb likes analogies heres one —
CO2’s carbonic acid is like oil tar, sulfuric acid is like gasoline.
Both tar and gasoline are oil based, carbonic and sulfuric are both acids.
Both tar and gasoline will burn, volume for volume, one is dangerously more flammable than the other. Likewise both carbonic and sulfuric acids are acidic, and molar concentration to molar concentration, one is more dangerously reactive than than other.
For seb, as he requires more remedial tutoring, READ HERE NOW! http://www.org-chem.org/yuuki/acid/acid_en.html and learn how comparatively weak is carbonic acid.
Looks like Professor Peter Ridd was right about the nonscience scare stories being produced about the Great Barrier Reef.
And the green fascists got him sacked.
Really? Google Scholar seems to be full of such studies …
https://aslopubs.onlinelibrary.wiley.com/doi/abs/10.4319/lo.2012.57.3.0698
https://www.nature.com/articles/nature07051
and so on
Umm, the driving mechanism is really simple physics. More CO2 in the atmosphere causes more CO2 to be absorbed by the oceans even though the oceans are supposed to warm even further (keyword is: differential pressure). These are pretty fundamental laws and yes, we do have measurements.
And yes, that is a model! Just as when I tell you that the temperature of your glass of Cola will reach about 0°C when you drop ice cubes in it or when I tell you that your car will drive 50 km in the next half hour if you maintain a speed of 100 km/h. These predictive skills are based on a model that is based on the laws of physics. Your aversion to models is weird …
P.S.: Cookies still not working. Is your DSGVO plugin acting up?
“the driving mechanism is really simple physics”
… which is the only sort you are capable of.
One day you may get to something more intellectual.. or NOT !!
There is ZERO evidence of enhanced CO2 having any affect on anything except plant growth. The so-called mechanism you are brain-hosed about are a pure fantasy based of mindless assumptions.. NOT PHYSICS
And yet more juvenile attempts at manically irrelevant analogies.
One day, you may figure out that your simplistic child-minded analogies show JUST HOW LITTLE you actually comprehend about anything.
But until then.. your clown act will suffice
OMG seb
your links.. did you really want to use them ??
Did you just pluck them out of la-la-land like you usual citings???
First one is NATURAL ocean upwelling of cooler more CO2 rich water. Stuff happens.
Second one is mindless AGW supposition, based on absolutely NOTHING of any sort of real science.
It uses every misdirection and anti-science nonsense they could come up with.
About the only thing it does show is that like land life, sea life is adapted to a wide range of conditions.. well… DURRRR !!!
But that is the AGW meme that you are stuck in, isn’t it, without the intelligence or guts to break out of your AGW -cult stupor !.
Please provide the physically-established link between anthropogenic CO2 emissions and the deleterious effect on aquatic life. Neither one of your links do that.
Umm, the explanation for why pH changed by -0.08 of a unit over the last 265 years (-0.0003 per year) but will change by -0.4 (five times greater) in 82 years (183 fewer years) is not explained. Please provide the mechanism that dramatically changes the rate of “acidification” by 2100 but has not changed it at that rate over the last 265 years.
So if they’re so fundamental and basic, why do scientists acknowledge that “critical mysteries remain” about the ocean as a net carbon sink, that we have an inability to quantify the “mean CO2 sink” observationally (all we have are models) and thus we do not have a “mechanistic understanding of how the ocean carbon sink works”? Furthermore, scientists admit that “it is not yet possible to directly confirm from surface observations that long-term growth in the oceanic sink is occurring” and that this is “sufficient to prevent detection of anthropogenic trends in ocean carbon uptake on decadal timescales”. In other words, why do real scientists disagree with you about how “fundamental” our understanding is of the oceans as a net carbon sink?
—
McKinley et al., 2017
http://www.annualreviews.org/doi/10.1146/annurev-marine-010816-060529
“That the growth of the partial pressure of CO2 gas in the atmosphere ( pCO2 atm) drives a growing oceanic sink is consistent with our basic understanding that, as the globally averaged atmosphere-to-ocean pCO2 gradient increases, carbon accumulation in the ocean will occur at an increasing rate (Section 3). This behavior has been illustrated clearly with models forced with only historically observed increases in pCO2 atm and no climate variability or change (Graven et al. 2012, Ciais et al. 2013). Nonetheless, critical mysteries remain and weigh heavily on our ability to quantify relationships between the perturbed global carbon cycle and climate change.”
“The current inability to accurately quantify the mean CO2 sink regionally or locally also suggests that present-day observational constraints are inadequate to support a detailed, quantitative, and mechanistic understanding of how the ocean carbon sink works and how it is responding to intensifying climate change.”
“This lack of mechanistic understanding implies that our ability to model (Roy et al. 2011, Ciais et al. 2013, Frolicher et al. 2015, Randerson et al. ¨ 2015), and thus to project the future ocean carbon sink, including feedbacks caused by warming and other climate change, is seriously limited.”
“The sum of the available evidence indicates that variability in the ocean carbon sink is significant and is driven primarily by physical processes of upwelling, convection, and advection. Despite evidence for a growing sink when globally integrated (Khatiwala et al. 2009, 2013; Ciais et al. 2013; DeVries 2014), this variability, combined with sparse sampling, means that it is not yet possible to directly confirm from surface observations that long-term growth in the oceanic sink is occurring. … [T]his CESM-LE analysis further illustrates that variability in CO2 flux is large and sufficient to prevent detection of anthropogenic trends in ocean carbon uptake on decadal timescales.”
Scientists disagree with you and your beliefs about the oceans as a net sink. Instead, they find the oceans to be a net source.
Reimer et al., 2013
https://agupubs.onlinelibrary.wiley.com/doi/abs/10.1002/jgrc.20319
“The study of air-sea CO2 fluxes (FCO2) in the coastal region is needed to better understand the processes which influence the direction and magnitude of FCO2 and to constrain the global carbon budget. The near-shore region was a weak annual net source of CO2 to the atmosphere (0.043 mol CO2 m-2 y-1); where 91% of the outgassed FCO2 was contributed during the upwelling season.”
—
Astor et al., 2013
http://reef01.marine.usf.edu/sites/default/files/project/cariaco/publications/Astor_et_al_2013.pdf
“Based on these observations, 72% of the increase in fCO2sea in Cariaco Basin between 1996 and 2008 can be attributed to an increasing temperature trend of surface waters, making this the primary factor controlling fugacity at this location. … An increase/decrease of 1°C is usually followed by an increase/decrease of 16–20 matm of fCO2sea. Thus, the SST increase of 1.3°C between 1996 and 2008 accounted for 16 matm increase in fCO2sea explaining around 72% of the fCO2sea observed variation. This suggests that the changes measured in fCO2sea were primarily the result of surface-ocean warming in Cariaco Basin. … These observations confirm that this area is a consistent source of CO2 to the atmosphere. The main process controlling the long-term changes in surface fCO2sea at CARIACO was temperature, with net community production playing a secondary role. … At the CARIACO site, the ocean is primarily a source of CO2 to the atmosphere, except during strong upwelling events.”
—
Ikawa et al., 2013
https://www.biogeosciences.net/10/4419/2013/bg-10-4419-2013.html
“We estimated that the coastal area off Bodega Bay was likely an overall source of CO2 to the atmosphere based on the following conclusions: (1) the overall CO2 flux estimated from both eddy covariance and pCO2 measurements showed a source of CO2; (2) although the relaxation period during the 2008 measurements were favorable to CO2 uptake, CO2 flux during this period was still a slight source; (3) salinity and SST were found to be good predictors of the CO2 flux for both eddy covariance and pCO2 measurements, and 99% of the historical SST and salinity data available between 1988 and 2011 fell within the range of our observations in May–June 2007, August–September 2008 and November 2010–July~2011, which indicates that our data set was representative of the annual variations in the sea state. Based on the developed relationship between pCO2, SST and salinity, the study area between 1988 and 2011 was estimated to be an annual source of CO2 of ~ 35 mol C m−2 yr−1. The peak monthly CO2 flux of ~ 7 mol C m−2 month−1 accounted for almost 30% of the dissolved inorganic carbon in the surface mixed layer.”
Comment here vanished?
One more try then:
You wrote that there were no studies about the effect of OA on marine life. I showed you that there are such studies. Now you are asking for a connection to CO2 emissions and more than enough comment authors here mentioned Henry’s law. So what is your problem?
Exponential growth Kenneth. It wasn’t a linear decrease of the pH levels (besides, the pH scale is not linear either, so 0.4 isn’t five times greater than 0.08 of a change).
Your papers:
How does this work? You post one that does a good job of explaining ocean acidification and clearly shows that the oceans as a whole are a net sink and then you dig up papers which (correctly) claim that some regions are net sources … and you don’t see the contradiction in claiming the later for the whole oceans?
Weird.
No, I didn’t write that. Instead, I directly quoted an oceanographer, who wrote this:
McElhany, 2017
https://academic.oup.com/icesjms/article/74/4/926/2669543
“[T]here are no studies that directly demonstrate modern day effects of OA [ocean acidification] on marine species. … The ocean acidification (OA) literature is replete with laboratory studies that report species sensitivity to seawater carbonate chemistry in experimental treatments as an ‘effect of OA’. I argue that this is unintentionally misleading, since these studies do not actually demonstrate an effect of OA but rather show sensitivity to CO2. Documenting an effect of OA involves showing a change in a species (e.g. population abundance or distribution) as a consequence of anthropogenic changes in marine carbonate chemistry. To date, there have been no unambiguous demonstrations of a population level effect of anthropogenic OA, as that term is defined by the IPCC.”
But, for that matter, there are paper that show decreased pH (ocean “acidification”) and warmer water actually is beneficial to aquatic species. So what does this demonstrate?
Hernandez et al., 2018
https://www.sciencedirect.com/science/article/pii/S0141113617306797
“Decreased pH [ocean “acidification”] had a positive effect on short-term production of the studied species. Algae with tropical affinity increased their production at higher temperatures. Respiration rates were higher at the lower temperature treatments. Future pH and temperature conditions benefit tropical algal species. … The results suggest that biomass and productivity of the more tropical species in coastal ecosystems would be enhanced by future ocean conditions.”
In what way is there going to be exponential growth in CO2 concentrations if it’s your simultaneous belief that exponential growth in wind and solar will supplant fossil fuel energy sources by 2100?
You wrote:
According to McElhany, 2017, “there are no studies that directly demonstrate modern day effects of OA [ocean acidification] on marine species.” There still aren’t.
And now you expand the “quote” with context because you found out yourself that out of context quotes aren’t telling the whole story? Hmm …
Sure, why not? Fungi also thrived during the big extinction events … more organic mass to digest.
You asked for the reason why the oceans should “dramatically” acidify. I didn’t reply that CO2 concentration would increase exponentially, that’s something you just made up to have another straw man.
Why do you keep doing this and simultanously call me dishonest all the time? It’s a super strange behaviour …
The quote was abbreviated entirely for the sake of brevity, as it was a subtitle. And, again, no, neither one of your two links “directly demonstrate modern day effects of OA on marine species.” Read what a direct demonstration of this would actually mean in the McElhany paper. You have, once again, failed to accurately respond to what was actually written.
Please provide the mechanism that dramatically changes the rate of “acidification” by 2100 but has not changed it at that rate over the last 265 years.
So then what does the “exponential growth” refer to? I’m asking why pH levels changed by -0.07 of a unit over the course of 265 years, but it’s predicted they’ll change by -0.4 in the next 82 years. What’s the mechanism for the abrupt rate change? Your answer: “Exponential growth, Kenneth.” In what? If not CO2 emissions (which you believe are 100% (?) responsible for the “acidification” change), then what mechanism is going to undergo exponential growth to elicit a dramatic change in pH?
Huh? I have no idea what else you could possibly be referring to that would cause the pH change…if not CO2 emissions. What else is there that you believe will cause the pH levels to plummet by -0.4 in the next 82 years even though they allegedly only fell by -0.07 in the last 265 years?
What was written is what is in the subtitle. You added “There still aren’t.” and no, the reader can not be expected to read a linked paper to exactly understand what you mean by that quote. You quoted this because it sounds good for your cause. Anyway, to quote your author: “I argue that this is unintentionally misleading, since these studies do not actually demonstrate an effect of OA but rather show sensitivity to CO2.” … well, he argues. Good for him.
Once again you lift the claim of one author against all the other authors and studies in the field and assert that this is what’s going on. Not a least bit skeptical, Kenneth?
There is no abrupt rate change. You are looking at these numbers and imagine a linear decrease of the pH value over 2+ centuries and then you imagine the decrease suddenly jumps much faster. I am sure there are some online calculators that let you calculate pH change from alkalinity, temperature and dissolved inorganic carbon. Here you go: http://biocycle.atmos.colostate.edu/shiny/carbonate/
Question: do you think the oceans already reached the pH value associated with a CO2 concentration of 400 ppm?
In everything. There is not much linear growth going on in all of the involved variables nor are the scales linear. Use that online tool above and set it to “modern”. Then increase the DIC amount to about 2160 uMol/kg to get the results equivalent to an ocean in equilibrium with a 560 ppm CO2 atmosphere (and no further temperature increase). Then increase it even further. Notice the non-linear ph scale going down.
Of course emissions, but they don’t need to grow exponentially to have “exponential growth” in the pH value. In fact just continued emissions as they are now will cause a pH decrease that is quite large. I assume you know the CO2 concentrations figures we will reach for different scenarios. Those values include the (yes, modelled) uptake of the oceans.
Interesting. Please cite a source that clearly demonstrates a robust and direct link between anthropogenic CO2 and severe disruptions to marine biota. Not implied. Not assumed. Not modeled. Not We think it might happen someday. A real, direct and causal link. Let’s see what you’ve got.
Durante et al., 2014
http://bioscience.oxfordjournals.org/content/early/2014/12/24/biosci.biu198.full.pdf+html
Therefore, there is, as yet, no robust evidence for realized severe disruptions of marine socioecological links from ocean acidification to anthropogenic CO2, and there are significant uncertainties regarding the level of pH change that would prompt such impacts.
[A] robust demonstration of a direct causal link between global warming and global coral bleaching over decadal time scales has not yet been produced.
Correct. Over the last 265 years, pH has been claimed to have changed by -0.07 in total. Over the next 82 years, pH is projected to change by -0.4 of a unit. Which is the faster rate of change, and how much faster is the faster rate than the slower rate? Is that rate change negligible, or is it significant?
Sea levels have been rising at a rate of about 1.4 mm/yr (~5 or 6 inches per century, or about a half an inch per decade) since the late 1950s. According to James Hansen (in a 2015 paper), sea levels will rise by 10 feet in the next 50 years (by 2065) due to Antarctic and Arctic ice sheet melt, which is 240 inches per decade). Do you think that there is a substantial or negligible difference between 0.5 an inch per decade and 240 inches per decade? Would a rate change from 0.5 to 240 inches per year be “abrupt”? If yes, what would the mechanism be for that abrupt rate change? Will you be answering by saying “Exponential growth, Kenneth” again?
Kenneth, the very paper you quoted the subtitle from states that he argues that all we have are studies about the effect CO2 has and not ocean acidification.
You are being weird now again?
Question: how much of the supposed 0.08 change (don’t know where you got 0.07 from) do you think happened in the first 100 years vs. the last 100 years of that 229 years (1765-1994) timespan? How much of the predicted change until the end of the century (-0.2 to -0.4 on the pH scale according to the IPCC report) do you think happens now vs. in the last 10 years of that timespan?
Current rate seems to be -0.0017/yr to 0.0024/yr. If it doesn’t increase further we will end up with up with a pH value 0.14 to 0.20 lower than today at the end of the century.
I hope you understand it now. If not, feel free to ask.
Correct. And the assumption there is that CO2 concentration determines the pH of the oceans. You, yourself, believe that, as you believe there is a pH value associated with 400 ppm (that we haven’t reached yet). Please do cite the value of the ocean’s pH based upon the air’s CO2 concentration.
(Did you not realize that this response validates what was written in the subtitle of the article, and renders your accusations baseless? Apparently not.)
So what year did you carefully select as your starting point in claiming that this is the “current” rate? Be specific, as I’m sure you chose that particular year because it supports your belief that the “trend” is clearly decreasing at a faster pace during the “current” time relative to the past.
If we were to start in the year 1979 according to this graph…
https://notrickszone.com/wp-content/uploads/2016/12/Holocene-Cooling-Pacific-West-SSTs-pH-Wei-15.jpg
…we’d get an overall slight pH increasing trend (less “acidification”) in the last few decades.
If we were to start in the year 1988 (the year with the highest pH value), we get an overall decline in pH, and thus it can be claimed that CO2 has been “acidifying” the oceans since 1988.
So what year did you select as your starting point, SebastianH?
By increase you mean decrease, right? Again, your estimate is quite flawed because you have arbitrarily decided upon what the “current” rate based on the arbitrary selection of a particular year. If you were to choose a different year as a starting point you’d get a completely different result. But we all know that this is exactly what the catastrophic anthropogenic global warming belief is based upon: the careful selection of starting points that confirm one’s presuppositions.
“Current rate seems to be -0.0017/yr to 0.0024/yr. If it doesn’t increase further we will end up with up with a pH value 0.14 to 0.20 lower than today at the end of the century.”
ROFLMAO..
seb takes a fabricated nonsense number, then extrapolates out to the end of the century.
A MANIC display of mathematical and scientific INEPTITUDE.
MINDLESSLY FACEPLANTING in your own BS, yet again, seb
Oh come on, are you really claiming here that the partial pressure differences between CO2 in the ocean and in the air doesn’t determine (together with temperature and alkalinity) the pH of the surface water? It’s not a believe Kenneth, that’s virtually certain. Just as the Earth not being flat is not a belief.
Check http://biocycle.atmos.colostate.edu/shiny/carbonate/ for simple equations. If you feel that is wrong, write them an e-mail and correct them.
It doesn’t.
I looked up the section of the IPCC report where they write about OA, since you seemed to be taking your numbers from there. Don’t remember that part of the paragraphs?
The rate increases when it changes from say -0.002 / yr to -0.003 / yr … or is that different in the English language? In any case I meant a increasing acidification rate.
And yes, if I would choose 1765 as the starting year I’d get a very small overall rate. Again, you didn’t reply to my question how much of the decrease of the pH you think happened in the first 100 years and in the last 100 years of that 1765 to 1994 timespan. Do you think those amounts are equal?
Regarding your graph: you seem to think that the 0.08 pH decrease that was calculated from the estimated CO2 emissions in that timespan is the only thing that happened in this timespan, right? Same as you think that 100% of the CO2 concentration increase being caused by humans doesn’t allow for natural variations at the same time.
@AndyG55:
I guess climate scientists made a big mistake not listening to your valuable input. Maybe you should become a IPCC report reviewer and help them to correct their huge mistakes that seem to slip through.
“do you think the oceans already reached the pH value associated with a CO2 concentration of 400 ppm?”
What do you think that pH reading should be for 400ppm, seb
Tell us and use real science that takes into account the MASSIVE buffering effect of ocean carbonates, of large concentrations of Ca, Mg and many other minerals that interact with CO2 and H2O
Also take into account massive use of CO2 by ocean plant life.
Tell us how, despite basically every river that flows into the oceans over millions of years has been below pH 7, often significantly, yet there was no change in pH until there was a tiny change in atmospheric CO2
Tell us how , taking all known sea surface pH readings over time, gives a ZERO TREND
https://s19.postimg.cc/ewnhcf8bn/ocean_PH_all_surface_readings.png
This question contains a hint to the answer already … I’ve highlighted it for you.
Apparently you have a figure in mind. Do you want to share or just yap at the person that tries to answer your requests?
AndyG55: What do you think that pH reading should be for 400ppm, seb
SebastianH: “Apparently you have a figure in mind. Do you want to share or just yap at the person that tries to answer your requests?”
Why would AndyG55 have a figure in mind since he clearly does not share your belief that there is a pH unit value associated with 400 ppm CO2 (that we someday will reach, but haven’t reached yet)? In what way is it even “apparent” that AndyG55 has a figure in mind?
ROFLMAO
Again you twist and turn away from answering a question that you said you= were able to answer.. BUT CAN’T
So PATHETIC, but exactly what we have come to expect.
seb seems to think that a tiny amount of CO2 is stronger neutraliser than MILLIONS of years of acidic sea water
What a BIZZARE, twisted, little fantasy mind you have , seb
EMPTY of all rational thought.
“Apparently you have a figure in mind.”
YOU are the one that is saying it should have a certain pH
“do you think the oceans already reached the pH value associated with a CO2 concentration of 400 ppm?””
STOP RUNNING AWAY FROM YOUR OWN STATEMENTS.
its PATHETIC.. but its what you do.
Come on seb..
What ocean pH value is associated with an atmospheric CO2 concentration of 400 ppm ?
With scientific, measured proof, not some AGW mantra nonsense non-science.
Kenneth:
Even if he really believes that CO2 does nothing to the pH value of the surface ocean water, he would have a figure in mind. I doesn’t really matter what I would calculate here, any number I say will be automatically false by some arbitrary reason, because it is not coming from a fellow skeptic.
It’s pointless doing math for you guys, you have to do it yourself and hopefully recognize your mistakes on the way …
@AndyG55:
Seriously, “millions of years” of acidic sea water and you don’t think that some kind of equilibrium would have formed? I have news for you, the Sun has been shining for billions of years and yet Earth hasn’t molten yet.
Use this handy tool to find out: http://biocycle.atmos.colostate.edu/shiny/carbonate/
Not going to do your homework to be yapped at afterwards.
“What ocean pH value is associated with an atmospheric CO2 concentration of 400 ppm ?
“
ROFLMAO
again, your manifest inability to answer a question which you said you have an answer for
ANSWER THE QUESTION, clown, and stop your COWARDLY squirming and evasion.
It makes you look even more PATHETIC than you already are.
What ocean pH value is associated with an atmospheric CO2 concentration of 400 ppm ?
You seem to think there is a set value.. WHAT IS IT !!
Is that even possible !!!
Poor seb
makes a statement then cannot back it up. then spends his time squirming and slithering to avoid answering
quite PATHETIC. !! but al he can do.
Come on seb, you say that there is a set pH for an atmospheric CO2 content of 400ppm.
WHAT IS IT ????
What is this MYTHICAL value you want to invent ??
WE are all WAITING for your less cowardly response.!!!!
I’m going to say its in the range pH 7.5 to 8.5
https://s19.postimg.cc/xoet6sn4j/ocean-ph-along-transect.jpg
just like it was at 300 ppm,
just like it will be when there is 500 ppm
and just like it will be when the atmospheric CO2 reaches 600ppm.
BUFFERED. !
“Seriously, “millions of years” of acidic sea water and you don’t think that some kind of equilibrium would have formed?”
Yes, and no tiny change in a minor trace gas is going make one iota of difference to that equilibrium. You have discovered BUFFERING.
Take another faceplant, seb
Its your natural position, face-down in your own BS. !!
The ridiculous thing is that from indirect measurements (via proxies) it can be shown that all marine life alive today have survived both more acidic/less acidic, higher salinity/lower salinity, and warmer/cooler temperatures than today.
The caveat is our lack of knowledge of the thousands (tens of thousands) of underwater volcanoes and how they influence affects ocean chemistry, pH, salinity, and temperature today and recently.
However it is obvious to any rational person’s perspective, that current atmospheric CO2 levels (at 400ppm) are too low to significantly affect the oceans in pH (CO2 levels have shown no effect on ocean temperatures to date).
Henry’s Law rules, even for seawater.
Why do so many so called ‘scientists’, deliberately choose to ignore the simple truth that life and evolution has ensured that currently we have *the best* of the survivors living on the planet now. Also all we know today about the oceans indicates that all recent variations in pH, temperature, and salinity appear to be safely within natural limits.
In respect of the oceans, just like the atmosphere, there is nothing remotely alarming going on.
The last sentence is the only correct assertion in this quote. At the current CO2 concentration about 5 GtC are getting absorbed by the oceans each year.
The simple truth is, that ocean pH could potentially be changing in a much shorter timespan than what evolution can cope with. If that happens the food chain will get disrupted significantly.
And yes, I am aware that pH fluctuates on very short timespans too. Your very own body will be totally fine to swim in 15°C water for an hour every day too, but you won’t survive swimming in 15°C water for 12-24 hours. Do you understand the problem?
“Do you understand the problem?”
Yep, you lack any understanding of chemistry and biology, and have to resort to analogies to show how little you know.
that ocean pH could potentially be changing in a much shorter timespan than what evolution can cope with
roflmao. what a load of hallucinogenic BS. !!
… evolution is how we got here.
And where is this FANTASY change in pH you keep coming up with.. No sign of it anywhere… (except in ASSumption driven models.)
https://s19.postimg.cc/ewnhcf8bn/ocean_PH_all_surface_readings.png
AndyG55, what caused the Permian-Triassic mass extinction?
And that took place over thousands of years, while the current change will only take hundreds of years if we continue with business as usual.
Rapid glaciation and sea level reduction from an ice age attributed to a decrease in absorbed solar radiation due to an uptick in volcanic activity.
—
https://www.upi.com/Ice-age-not-warming-explains-Permian-Triassic-extinction-event/6871488815458/
“Analysis of the newly dated layers showed a significant reduction of seawater levels during the extinction event. The only explanation for such a dramatic decrease in water levels is a sudden increase in ice. The ice age lasted just 80,000 years, but the extreme cold was enough to kill off the majority of marine species. … [A]n uptick in volcanic activity and the release of sulphur dioxide into Earth’s atmosphere blocked out the sun and triggered the brief ice age.”
—
But what are your beliefs as to what caused it? CO2 concentration changes?
As suspected. You believe CO2 concentration changes caused the extinction, and that’s why you believe we’re facing a catastrophic mass extinction event if we don’t start using more wind and solar. What does your belief system say about what caused the CO2 concentration changes during the Permian-Triassic?
This is a probably a good time to remind the uninitiated that SebastianH actually believes that 30,000 extinctions are happening every year. This is the person who claims that we are the “disinformers” because we don’t ascribe to his CO2-induced catastrophic beliefs.
https://notrickszone.com/2017/10/16/recent-co2-climate-sensitivity-estimates-continue-trending-towards-zero/#comment-1232607
SebastianH: “Regarding extinction of species, why do you think 30,000 species lost per year is a big number? We are already at or over that rate.”
“while the current change will only take hundreds of years if we continue with business as usual.”
What a load of mind-numbed fantasy !!
Get back to the REAL world seb.
CO2 is a NECESSITY for ALL life on Earth.
EVEN YOU !!
And the pre-industrial levels were pretty much at plant subsistence levels.
Only a DECREASE in atmospheric CO2 level can do any harm to the planet.. by stopping plant growth.
Increase is ABSOLUTELY and TOTALLY BENEFICIAL at any level it is likely to get in the next millennium or more.
There is ZERO evidence that enhanced CO2 has anything except beneficial effects on the planet’s atmosphere or biosphere.
Why do you choose to remain so wilfully IGNORANT about LIFE on this planet, seb.
You should probably read the paper they are referring to and do a quick search for the word “acidification”.
You probably also know that the continents looked vastly different 250 million years ago and that there are quite a few theories of what has happened back then. But you chose the one most convenient for you (and leaving out the part about acidification … one wonders why).
Nope, ocean acidification. And yes, the CO2 concentration increased a bit back then: https://www.sciencedirect.com/science/article/pii/S0009254112002938
My belief system? What does your belief system say? Science says volcanic erruptions caused a rapid release of CO2 and CH4. CO2 levels reached 1400 to 3000 ppm.
Sorry Kenneth, you are becoming increasingly weird and anti-science. Only seeing what you like to see and applauding claims from other comment authors that couldn’t be more wrong, but feel right to you.
Yes, indeed. Look at the paper. An abrupt cooling/ice age event caused the mass extinction. In the paper, the “acidification” is not caused by CO2 changes, but by “sulfur volatiles” from the extensive volcanism.
https://www.nature.com/articles/srep43630
“In adjacent deep marine troughs, rates of sediment accumulation display a six-fold decrease across the PTB compatible with a dryer and cooler climate as indicated by terrestrial plants. Our model of the Permian-Triassic boundary mass extinction (PTBME) hinges on the synchronicity of the hiatus with the onset of the Siberian Traps volcanism. This early eruptive phase released sulfur-rich volatiles into the stratosphere, thus simultaneously eliciting a short-lived ice age responsible for the global regression and a brief but intense acidification. Abrupt cooling, shrunk habitats on shelves and acidification may all have synergistically triggered the PTBME.
“Subsequently, the build-up of volcanic CO2 induced a transient cool climate whose early phase saw the deposition of the microbial limestone.”
“Stratospheric injection of volcanogenic sulfur volatiles and subsequent condensation into aerosols are seen as the proximal cause for brief climate cooling and ensuing global regression, provided that the cooling was profound enough to store water as terrestrial ice. Sulfur volatiles also provide a direct mechanism for ocean acidification. Abrupt cooling likely resulted from the atmospheric injection of both volcanogenic and remobilized SO2 and H2S from early Paleozoic evaporites by the initial emplacement of dykes and sills of the Siberian Traps. This scenario simultaneously accounts for acidification of ocean surface waters and for the global regression. The synergistic effects of shrunken marine habitats on continental shelves through a global eustatic regression [sea levels falling], of fast temperature drop down, and of substantial acidification are all compatible with the new timing proposed here.”
Uh, what caused the acidification according to the paper, SebastianH? Was it CO2 concentration changes or sulphate pollution from the volcanism? (I’ll answer: the latter.)
And if CO2 concentrations are causing warming, and the mass extinction event was caused by an extensive cooling event that lowered sea levels (which by itself makes waters less alkaline) and covered the land masses with ice sheets, how is this even remotely analogous to what is claimed by proponents of catastrophic global warming (like you) to be happening today due to fossil fuel emissions?
So then why was there an abrupt cooling/ice age that lowered sea levels if the CO2 concentration increased?
Again, if the CO2 concentration reached 1400 to 3000 ppm during the Permian-Triassic extinction event, why was the Earth covered in ice as the sea waters plummeted to ice age levels (-120 meters)? Or did the CO2 concentration rise cause the cooling and sea levels to drop?
Umm, Kenneth … that is their theory. All the other theories about this time period don’t claim that there was an ice age.
“According to the paper”. Why use the paper’s content as an argument against you using this paper’s content because it confirms your bias? Weird.
Ehm, why do you think it was caused by extensive cooling? Right, because this particular paper says so 😉
Haven’t you read a single competing theory of what happened back then?
Was there? You seem to be confusing facts and theory. Can an ice age explain why this extinction affected organisms with calcium carbonate skeletons more than others? Can an ice age explain why it took millions of years before recovery even started?
I’ll stick to the other theories. Volcanism and/or large impact, CO2/CH4, ocean acidification, fast rise in temperature and unfriendly conditions for life for a long time. As for sea levels, I don’t know, but there are papers claiming that they increased back then. But who knows, after all Earth looked very different in the times of Pangaea and the Superocean.
Yes, indeed. Look at the paper. An abrupt cooling/ice age event caused the mass extinction.
Um, SebastianH, they’re not the only scientists who attribute the Permian extinction to the onset of an ice age and plummeting sea levels. You’re going to need to do better than claiming that the Baresel et al., 2017 paper that says there was a “significant reduction of seawater levels during the extinction event” is something of an outlier. Because it’s not. The Permian extinction coincided with fatally low sea levels and global cooling, as just published papers affirm…
https://notrickszone.com/wp-content/uploads/2018/05/Cooling-and-Sea-Levels-100-Meters-Lower-Than-Today-Caused-Permian-Mass-Extinction-Kani-2018.jpg
https://notrickszone.com/wp-content/uploads/2018/05/Cooling-Permian-Extinction-Low-Sea-Levels-Isozaki-Servais-2018.jpg
Kani et al., 2018
Nonetheless, the sea level during the Capitanian [Permian] contradictorily recorded the lowest stand of the Phanerozoic (Haq and Schutter, 2008), suggesting a global cooling instead. Ice coverage and/or the predominance of arid climates under cooling during the Permian likely accelerated the decrease in the seawater Sr ratio.
In general, the termination of shallow marine carbonates may occur with a decrease in the seawater temperature. Two possible causes can be inferred for the late Capitanian temperature decrease, i.e., 1) the appearance of global cooling, and 2) the migration of depositional sites to higher latitudes with cooler climates.
[I]n a global summary of sequence stratigraphy (Haq and Schutter, 2008), the findings of coeval glacial deposits in eastern Australia and in Mongolia (Fielding et al., 2008; Fujimoto et al., 2012), the selective extinction of tropical fauna (Isozaki and Aljinovic, 2009), the migration of mid-latitude fauna to low latitudes (Shen and Shi, 2002), and the Milankovitch tuning (Fang et al., 2017), all support the onset of cooling in the Capitanian [Permian].
—–
Isozaki and Servais, 2018
Besides the similarity in extinction patterns with the preferential elimination of particular clades, we can recognize a major resemblance but of non-biotic nature between the [Ordovician] Hirnantian and [Permian] Capitanian [mass extinction] events, that is the significant cooling coupled with global sea-level drop.
A sea-level drop of nearly 100 m can be achieved solely by transferring vast seawater onto land in the form of ice. It is noteworthy that sea level was much higher in the Late Ordovician (ca. 60 m above the present-day level) than in the Middle Permian (ca. 80 m below the present-day level), even after the sea-level drop in the same magnitude.
The evidence for the late Capitanian [Permian] global sealevel drop (for up to 100 m) is robust. … The sharp erosion of reef limestone in low-latitude mid-ocean implies a large eustatic sea-level drop, in other words, the appearance of a global cooling.
The biotic responses in the Hirnantian and Capitanian appear compliant with all these lines of evidence for cooling, in particular, the latitudinal contraction of faunal distributions towards tropics together with the preferential elimination of preexisting tropical fauna. Regardless of the second Hirnantian episode, the first decline in biodiversity in both cases occurred during global cooling. In general, a relative drop in seawater temperature, particularly in shallow seas, is critical for the metabolism of almost all contemporary marine organisms. The updated lines of evidence therefore confirmed the classic notion of a putative link between the global cooling and extinction (e.g. Stanley 1988) for both cases [Ordovician and Permian mass extinction events].
“The simple truth is, that ocean pH could potentially be changing in a much shorter timespan than what evolution can cope with. If that happens the food chain will get disrupted significantly.”
Yet again more drivel from seb!
What scientific basis could any rational person ever come up with such hogwash!
Beyond the usual delusion, a completely mad idea.
“ocean pH could potentially be changing in a much shorter timespan”
HAhahaha, LOL!!!! Completely nuts!
Go on, seb, calculate just by how much the oceans could change in pH if ALL 400ppm of atmospheric CO2 was dissolve in the oceans,(hint it’s a piffling amount), no even sane to consider it will overstress marine life. I’m pretty sure you can not do the calculations (without looking up the answer) but if you can publish it here, and see how silly you are.
Do me the favor of calculating this for us. I want to see your math and “see how silly you are” 😉
Besides, it’s not possible for the oceans to absorb all the CO2 of the atmosphere because then the partial pressure of CO2 in the atmosphere would be much lower than in the oceans.
Here is a simple online tool to calculate pH from DIC, alkalinity, and temperature: http://biocycle.atmos.colostate.edu/shiny/carbonate/
I am sure you know how many uMol of carbon would dissolve in the oceans if all the carbon in the atmosphere could dissolve.
So funny,
seb evades, squirms, oozes away around showing his ineptitude….
Its almost he KNOWS that atmospheric CO2 has NO MEASUREABLE EFFECT on ocean pH and just playing his usual mindless troll games.
Funny sea water in that app, seb
No Cl, no Na, no sulphates, no Mg, no CA, no LIFE…
Must be theoretical sea water.
YOU CAN’T DO IT!
And yet you have the temerity to pontificate that all this is basic science.
SCIENCE THAT YOU CAN’T DO!
[snip]
No one should pay you any notice on scientific matters as you are an empty void. YOU DO NOT KNOW HOW TO CALCULATE IT so stop prtending you know the answer. YOU KNOW NOTHING — A SCIENTIFIC BLANK!
DISINFORMER AND PROPAGANDIST FABRICATOR IS ALL YOU ARE!
[snip]
[snip][snip]
tomOmason, by you ranting reply I must assume you are the one who doesn’t know how to calculate it.
Please refrain from just mirroring accuses back. You are the disinformer and have always been. And I don’t need to write in all caps to make that clear … you are doing a fine job of demonstrating that yourself.
“Please refrain from just mirroring accuses back. You are the disinformer and have always been”
From you, that is HILARIOUS.
You are immediately using a mirror to AVOID producing anything.. its high deceit and quite disgusting behaviour.
Your pathetic “victim” act isn’t going to fool anyone here except yourself, after your continued twisting , turning and outright deceit over a long period of time.
You are a chronic ignoramus, incapable of supporting anything you say with anything resembling real science.
NOTHING but an attention-seeker who uses lies and denial of your own words as a sort of crutch of your manic ineptitude.
Have you or tomOmason ever produced anything here? When asked about details you always evade. I did the work in the beginning and realized it is futile with you guys. You just shut down and escape into insulting replies.
I am not here to do your homework. Just as you claim to be skeptics of established climate science, I am skeptic about your claims and you need to prove them and show that you understood how stuff works.
It surely isn’t you guys who are the victims of the great climate science and renewables conspiracy … right? 😉
The EMPTY yapping seb
You produce NOTHING to counter, when you have tried to you have been shot down because all you have is fantasy unsupported by anything except fallacy.
Asked hundreds of time to support the very basis of your cult-like “belief”
AND YOU CANNOT DO IT.
All you have EVER put forward has been erroneous ANTI-SCIENCE AGW propaganda BS..
Stop your childish wimpering victimhood.
NOBODY CARES about your opinions or feelings.!!
“and you need to prove them and show that you understood how stuff works.”
You have shown that the REALITY of how things work is totally and absolutely BEYOND your limited understanding of ……… everything !!
Your twisted ideas of reality come from a warped anti-science fantasy land.
As for proof… You are EMPTY !!
@SebastianH 28. May 2018 at 1:30 PM
No seb I was not ranting, I was laughing at you!
All your empty pontification and nothing, you attempt to distract away from my basic question (just as I knew you would), because you can not do science. The calculation (with appropriate approximations) are way beyond you.
You have proven yourself to be scientifically empty, everyone here sees how vacuous your response to my challenge has been.
AndyG55 has said it, and now I’ve shown it you are a self appointed disinformer in science. A perverter of education.
P.S.
I put those [snip] and CAPITAL LETTERS to arouse your natural pomposity and it worked.
You’re nothing but a vacuous vestibule of overbearing hubris.
Ok fine, let’s assume they are beyond me. Please demonstrate that they aren’t beyond you. Can you do the math? Prove it! I am calling your bluff …
Frankly, the only thing you are showing is how weak your arguments are.
Great … welcome back to Kindergarten tomOmason. I don’t know why I put up with this childish behaviour of yours.
Seb shows yet again, that he has absolutely ZERO scientific basis for anything he says.
He CANNOT back up his lies and disinformation with anything except more lies and disinformation, with added squirming and wimpering
A truly EMPTY mind, has seb, apart from the oozing green slime left by the AGW brain-hosing.
Henry’s law DOES NOT hold in toto for seawater! Henry’s only works when there are NO secondary chemical reactions which your very argument of Carbonic as well as other reactions will not give you a mass balance ( at least not the way you want). What the Keq of carbonic acid in seawater. I assure you Le Chatelier’s is driving more than Henry.
Nearly every river flowing into the oceans over many millions of years has been on the acidic side of neutral (some have been measured as low as pH 5.5)
And yet the oceans remain steadfastly around ph 8.1 or there abouts.
It takes particularly fevered or green-sludge-hosed mind to IMAGINE that a tiny change in an atmospheric trace gas will in any way affect the ocean alkalinity.
The whole idea is just moronically farcical.
But “believers” will always just “believe” because their gullibility and nil-education leaves them no choice.
Henry’s Law rules, even in seawater.
Tom, only for Nitrogen and Oxygen!
Not for Co2 because it chemically reacts with the water!
https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Physical_Properties_of_Matter/Solutions_and_Mixtures/Ideal_Solutions/Dissolving_Gases_In_Liquids%2C_Henry's_Law
John Brown, thanks for your input. I didn’t realize that Henry’s Law doesn’t fully apply to a CO2/seawater solution, since the CO2 reacts to form hydrated carbon dioxide and carbonic acid. Below, Mark Pawelek demonstrates that the carbonic acid is minute– .00015%.
I don’t know about the hydrated carbon dioxide. Would be interesting to know.
And think of all those little sea plants.
ALL using and giving off CO2, dependent on all sorts of things.
And the ocean currents, upwelling of more CO2 rich waters.
Anyone who thinks Henry’s law is anything but a small part of the balance, just isn’t paying attention.. Is UNAWARE. !!
An interesting study done during some ocean passages.
four different cruises, measuring both the atmospheric CO2 levels and at the same time, the amount of CO2 in the surface seawater.
Results shown here
https://s19.postimg.cc/9t6x0ausz/sst-vs-co2.jpg
1. Many parts of the ocean are 50 ppmv lower than the CO2 concentration of the overlying air, and many other parts of the ocean have 50 ppmv or more of CO2 than the CO2 in the air above.
2. the change is not only the size but even in the sign of the lowess curve connecting temperature and CO2 Compared to the CO2 level in the air, below about 17°C the seawater CO2 decreases with increasing temperature, at a rate of about -2 ppmv per °C.
3. Above about 17°C, however, the seawater CO2 content relative to the air increases fairly rapidly with temperature, at about +4 ppmv per °C.
To put it another way another way, when the water is cool, it contains less CO2 than the overlying air … but when the water is warm, it has more CO2 than the overlying air.
There is obviously something quite interesting going on.
Now.. who still thinks that Henry’s law is the major driving force of CO2 ocean/air interchange.
Also consider the fact that SST tend to set the temperature of the air directly above the surface, so a temperature differential generally does not exist.
John Brown 27. May 2018
Quite correct however —
“Hence, the majority of the carbon dioxide is not converted into carbonic acid, remaining as CO2 molecules. In the absence of a catalyst, the equilibrium is reached quite slowly. The rate constants are 0.039 s−1 for the forward reaction (CO2 + H2O → H2CO3) and 23 s−1 for the reverse reaction (H2CO3 → CO2 + H2O).”
From https://en.wikipedia.org/wiki/Carbonic_acid#Chemical_equilibrium
And that passage starts with “When carbon dioxide dissolves in water it exists in chemical equilibrium with carbonic acid:[4]”
Where [4] is the reference — Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 310. ISBN 0-08-037941-9.
And as far as I’m aware with pure water at normal atmospheric pressures and at room temperature, CO2 is quite readily soluble in water where over 99% exists as the dissolved gas, and less than 1% as carbonic acid (H2CO3).
In other words the CO2 is dissolved in water and then the (slow) reaction takes place for a very small proportion of the gas, now what approximately determines how much CO2 is dissolved in water, and how it dissociates from it?
Thanks for your follow up. Normally do not like Wiki, but will have a read and put the filter on to avoid being burned by potential anti science propaganda.
The Henry number for CO2 as gaseous phase in water is very little if compared to O2 and N2.
This still leaves the CO2 to enter into the watery phase. And as quoted in various citations of Henrys law, it does not apply to gases that react with the water to form an acid for example.
Your example is valid for pure water but from the change of the pH for rain it seems the CO2 does dissolves into the water and only very little will stay in gaseous form. Interesting is the time it takes to form the acid.
Henrys law is part of the explanation how CO2 gets into the water, but when it comes to judging how much CO2 is in the water, it is far less trustworthy due to the dissolution.
One main difference to the shown reactions with pure water is:
sea water is a buffered solution which will impact on the forming of carbonic acid. But it will not impact on the ability to hold come gaseous CO2. If that makes sense!
Sorry tom not for chemically reactive molecules such as CO2. Henry’s is for ideal (nonreactive) gasses. O2 and N2 follows henry’s but CO2 is a more complex gas law. ( retired physical chemist)
Tom 1
CO2 is a more complex gas law. Agreed, however (as far as I understand it) the overall effect of the reaction is only to alter the speed of the flow of the CO2 in and out of solution, and not greatly impact the volume.
What cAGW advocate propagandize as skeptics’ ‘aversion to models’ is as always nonsense.
All to often in climate research models are use inappropriately, and what is criticized is —
1/ The use of models as a replacement for actual observation and measurements.
2/ The use of models stuffed to bursting with inappropriate and unreliable assumptions.
3/ The ‘belief’ that incremental variations in modeled ensemble runs will give reliable and realistic results when the natural process being modeled it not fully understood.
4/ The use of models as representative of a complete natural process when no one knows how the process works, and no verification on the modeled results is done.
Models when used appropriately are extremely useful, however the IPCC inspired predictive climate models are just non-scientific junk propaganda tools and a perfect display of ‘state of the art’ fantasy virtual-reality modeling.
No amount of ‘tweaking’ will improve these climate models as their very operating assumption (that CO2 causes warming) and many other built-in presumption are just guesswork and not based on observations or measurements.
tomomason, scientific models are like computerized voting in America: It might be accurate, but blind faith about what takes place in a black box is unwarranted.
Thanks for deconstructing climate modelling based on its typical errors. The empirical is hardly represented at all, is it?
Maybe I had too much carbonated mineral water and the acid is rotting my mind, it is after all such a powerful acid (not).
And the unscientific individuals of the world push that very high levels of dissolved CO2 is dangerous — those unfortunate anti-science types believes it forms such a powerful acid — they are wrong!
To which I say to them (the cAGW fabricators) stop drinking carbonated mineral water and go back to the real thing, which because the CO2 is so strong, phosphoric acid is added — as that make so much sense, eh seb? (Not that you could possibly understand basic chemistry).
See the alarming page of https://en.wikipedia.org/wiki/Carbonic_acid to see how very powerful CO2 in pure water is.
Note well the line “Carbonic acid, which is a weak acid, forms two kinds of salts: the carbonates and the bicarbonates. In geology, carbonic acid causes limestone to dissolve, producing calcium bicarbonate, which leads to many limestone features such as stalactites and stalagmites.”
and
In nature —
“Addition of base to an excess of carbonic acid gives bicarbonate (hydrogen carbonate). With excess base, carbonic acid reacts to give carbonate salts.” That is happening continuously in the rivers and oceans.
Also see the ever helpful https://www.engineeringtoolbox.com/gases-solubility-water-d_1148.html for more on information Solubility of Gases in Water.
Yep, the ever helpful websites that put on a very helpful note:
If you thought that CO2 graph there is only dependend on temperature, you thought wrong.
As usual seb ‘believes’ he knows what other people think. Only [snip] do that, and seb has proven himself to be a very pompous one.
Seb appears unable to navigate a website but that is what I expected of one with such little curiosity or intelligence, for seb it appears there is only the edicts of the IPCC anti-science mantra to live by.
I’ll make it plain, I have offered a start for the fearful of science like seb, so read on, investigate further (there are plenty of links there), or stay an [snip] lost in the science that is well beyond your understanding, for ever deluded!
How does the solubility of air vary with temperature and pressure? What is a Prandtl number?
Unlike Kenneth I do not spoonfeed anyone with THE answer, as that teaches you nothing (and also in all science, unlike religion, is mostly an incomplete catalog of approximations and not utter certainty) — you have to make up your own mind. If you are curious and intelligent enough (and like seb many aren’t) you will find out, however many people are like seb, and can not self educate as they are neither intelligent nor curious enough, preferring to just recite the IPCC’s inspired religion and it’s deformed version of reality.
Seb likes analogies so here’s one —
“You can take a mule called Sebastian to water but you can’t make him drink.”
DNFTT
Isn’t there an e-mail between some of the climate anointed expressing frustration because of the failure to discover the missing human acidification footprint, and the *travesty* which has ensued as a result?
That’s Trenberth. And it’s about the inability to find warming.
“The fact is that we can’t account for the lack of warming at the moment and it is a travesty that we can’t.”
Kenneth, we’re aware of the travesty the climate cabal’s failure to find the strangely evasive hidden heat. That was my point.
Wouldn’t you reckon that deep down there are equally grave travesties regarding the lack of evidence for:
– decreasing pH in oceans?
– increase in the rate of GMSL rise (aka acceleration)?
– declining polar bear population?
– “climate” refugees?
– increase in weather disasters?
– shortage of oil, gas or coal reserves?
– a “tipping point”?
– tangible benefits of so-called “Climate Policy”?
– any detectable effect on human CO2 emissions, let alone “climate”, as a result of costly efforts to promote so-called “renewables”?
-…
-…
I reckon.
https://s19.postimg.cc/6boke7uk3/Little_orange_guy.jpg
Theys got nuffin’ !!
Apparently, my comment got deleted here.
Trenberth was referring to the lack of our ability to measure the exact heat flux. The stuff we do measure (again, not covering every spot/pathway) falls short of the amount of heat accumulation that should occur from the imbalance. And yes, we do measure an accumulation. He didn’t mean that it wasn’t warming.
But then again, you are skeptics. Pulling words/sentences out of context to build up a straw man is what you do …
He was referring to his inability to concoct data to prove his warped theories.
Its a TRAVESTY that seb is TOTALLY UNABLE to present one bit of empirical science showing atmospheric CO2 warms anything, anywhere, anytime
Its a TRAVESTY that he is TOTALLY UNABLE to show any change in ocean pH except for small parts of natural cycles.
Travesty” definition
“a false, absurd, or distorted representation of something”
in seb’s case, of EVERYTHING.
synonyms:
misrepresentation, distortion, perversion, corruption
…… seb, by definition.
SebastianH also believes that “Hide the Decline” did not mean that they were trying to hide the post-1960s temperature decline from proxy evidence. So it’s natural he also believes that he can explain away the Trenberth quote about not being able to explain the non-warming with CO2 rising.
Let us consider a few ‘basic’ facts:
1) When CO2 dissolves in water it can form carbonic acid, H2CO3. But that only happens for 0.3%, or so, of the CO2. The remaining CO2 stays as it is. Only a small fraction of dissolving CO2, 0.3%, is called an “acid”: CO2 + H2O H2CO3
2) Carbonic acid: H2CO3, can ionize to form acidic hydrogen ions (written as H3O+ (hydrated hydrogen ions). This happens to only a small propoportion of the carbonic acid (about 0.5%): H2O + H2CO3 H2O+ + HCO3-
3) 0.3% × 0.5% = 0.003 × 0.005 = 0.000015, 15ppm, or 0.0015%. Only 15ppm of dissolving CO2 has an “acidic” influence.
4) Here are some measures of dissolved mineral content of oceans: https://web.stanford.edu/group/Urchin/mineral.html
5) Ocean pH is dominated by dissolved bicarbonate and carbonate, both of which are basic. Carbonate being very basic. The ratio of bicarb to carb is about 9:1
6) The Henderson–Hasselbalch equation explains how to calculate pH of a buffered system, such as the oceans: https://en.wikipedia.org/wiki/Bicarbonate_buffer_system#Henderson%E2%80%93Hasselbalch_equation
7) Armed with what I just told you, everyone here should be able to calculate the effect on ocean pH of burning 500 Gt of fossil fuel since the beginning of the industrial era. You’ll find the effect negligible (a change of less than pH 0.001). Well outside the error bounds of measuring ocean pH.
8) NOAA claim that climate change has altered ocean pH making it “30% more acidic”. This is nonsense, and impossible.
Apologies for the broken equations. I think they should be:
CO2 + H2O ⇋ H2CO3
H2O + H2CO3 ⇋ H2O+ + HCO3-
Interesting.
Mark,
solubility and dissociation of CO2 in water are two different processes.
Solubility of CO2 in water is much less than that of O2 or N2.
http://butane.chem.uiuc.edu/pshapley/GenChem1/L23/web-L23.pdf
Your first statement is off, while I fully agree that humans will not be able to burn enough coal to change the oceans surface waters.
Mind everyone the deep see water is already acidic past the carbon compensation depth. http://www.gly.uga.edu/railsback/Fundamentals/OceanSolutes18IIIa.pdf
“Solubility of CO2 in water is much less than that of O2 or N2.”
I rather over-egging the pudding, solubility of CO2 is not radically different from O2 or N2.
See http://butane.chem.uiuc.edu/pshapley/GenChem1/L23/web-L23.pdf
Hmm, you probably confused a few percentages here. 0.3% is on the low end of the amount of CO2 that ends up as H2CO3 (very cold water?), but the other products don’t come out of this pool, they exist in addition to H2CO3.
So no, 15 ppm is just plain wrong.
That is not “interesting” as Kenneth proclaimed, it’s wrong.
The mentioned pH change of 0.1 on the scale is a 30% change.
“The mentioned pH change of 0.1 on the scale is a 30% change.”
And requires a further 1800% (or thereabouts) to become neutral
Also totally un-measurable over that time period. we can’t even measure the whole ocean pH to 1 dp even now.. and the mentioned change is a TINY fraction of the actual range of variability.
It is TOTALLY MEANINGLESS.
seb… perfecting the art of the faceplant !!
See, that is what Trenberth meant by not being able to measure the warming (completely). We have to interpolate and estimate since we can’t have sensors for pH (or temperature) on every square millimeter of the planet.
Why would it need to become neutral? It’s not like ocean acidification means that the oceans (at the surface) are actually becoming an acid. Just like greenhouse theory doesn’t mean that there is a glas roof somewhere in the atmosphere. You guys are taking this too literal while at the same time freely interpreting science to fit your belief system. Weird …
So you admit that the words used are NOTHING to do with REAL SCIENCE..
Yes, seb.. we already KNEW that.
.. just like the rest of the AGW meme.
ZERO match to REALITY !! FANTASY-SCIENCE.
Do you really think something can “acidify”, from being alkaline without going through pH7.
Your ignorance is becoming legendary !
Your every comment is becoming a TRAVESTY !
Take another faceplant, seb.
Its your natural position in this world.
“We have to interpolate and estimate since we can’t have sensors for pH (or temperature) “
ROFLMAO.
So you now also ADMIT that most of the data is fabricated, NOT REAL.
FACEPLANT.. seb !!
So hard to keep your BS stories straight, isn’t it. !!
I’ll admit that you seem to have nothing to do with science.
Yes, because the pH scale is just that, a scale for the activity of hydrogen ions. There is no zero point at 7, it is a continuous scale. Therefore it’s perfectly fine to call a change from pH 10 to pH 9 acidification. Why? Because the activity of the hydrogen ions moved into that direction.
That’s usually only a problem for those who invent stuff to confirm their bias. Making it consistent is difficult, as can be seen by your comments every day. You even have to resort to going all caps and minor insults to convince yourself that you are the one who tells the truth.
There should be studies about pseudoscientific “skeptics” and the way they behave in blogs/forums. I am sure 80+% is just an angry mob … why are you so angry, AndyG55?
“Therefore it’s perfectly fine to call a change from pH 10 to pH 9 “
Only if you want to use it for propaganda, NOT SCIENCE
Science uses the word neutralisation.
Seb doesn’t use SCIENCE words,
he uses PROPAGANDA words,
because that is all he knows.
And seriously, still using the pathetic “I’m a victim, holier than thou” card as part of your insipid , slimy troll-routine ???
How pathetically laughable you have become.
“That’s usually only a problem for those who invent stuff to confirm their bias.”
Which is EXACTLY what you spend your time doing.
You live in some sort of weird ANTI-SCIENCE fantasy land , where measurements and real science are trumped by manic brain-hosed, hallucinogenic-type fantasies.
As such, you just keep faceplanting post after post after post as your fairy-tales unravel.
Its now happening so often that you don’t even have the brain capacity left to realise you are doing it.
Its HILARIOUS to watch.
Please keep going. 🙂
You are a great advertisement of all that is WRONG and GULLIBLE in the AGW cult of climate non-science.
A New interest for Sebastian perhaps.
Dear Sebastian it might be a good idea to broaden your interests or you may just. maybe, sound narrow and prejudiced!
http://audiolatinproverbs.blogspot.co.nz/2007/01/si-tacuisses-philosophus-mansisses.html
Stultus quoque, si tacuerit, sapiens reputabitur,
I would be interested in the blog articles in reference to past climates but interesting discussion is lost inevitably in your attacks.
Maybe they are paid attacks.. they look like it.. but anyway they have lost credibility by repetition and are giving you a bad reputation on the internet.
Our seawater at the current temperature & atmospheric pressure must already be saturated with CO2– and therefore be impervious to external CO2, even if manmade.
The evidence: A slightly increased temperature causes seawater to give up some CO2. If it were still lacking in CO2 saturation it could not do so.
Therefore only a decrease in temperature or increase in pressure will cause available CO2 to enter into seawater. The mere existence, or increased concentration, of external CO2 cannot alter the relation of solvent and solute.
Note to Seb: Additions to CO2 don’t increase atmospheric pressure, only the percent of it which is supplied by CO2. Atm pressure is regulated largely by gravity and atmospheric mass.
Penelope.
The pressure seb is trying to explain is the diffusion pressure.
Its to do with gases always trying equilibrate their “partial” pressures between different substances.
The ability to hold certain concentrations of any gas varies between oceans and the atmosphere. Temperature, concentration, atmospheric pressure, partial pressures of other gases, surface upwelling all play a part, and it needs accurate measurements to decide which way gases are going, in or out of the surface of the water.
Add to that the general expansion of the biosphere, including the VAST marine biosphere, with its hunger for usable CO2, and the whole thing becomes rather complicated
Anyone who uses just the partial pressure of CO2, or just temperature etc to decide which way gases are flowing, is very much yapping up the wrong tree.
Seb, note that according to Henry’s Law, each gas within a mixture dissolves in liquid as if it alone occupied the entire volume of the original mixture. i.e., NOT according to its concentration in the mixture.
Applying this principle: the concentration of CO2 in atmosphere doesn’t determine the amount dissolved in seawater.
When the concentration of one gas in a mixture doubles, the volume increases accordingly. The total volume of the gas mixture increases too, but since we are talking about a trace gas one can safely say that partial pressure of CO2 is more or less directly proportional to the CO2 concentration in the atmosphere.
Of course it does. It’s not the only variable – I’d never thought I write this, but read AndyG55’s reply above … he is not wrong.
“the concentration of CO2 in atmosphere doesn’t determine the amount dissolved in seawater.”
It is one SMALL factor.
It certainly DOES NOT determine the interchange of CO2 between atmosphere and ocean
Penelope is TOTALLY CORRECT in her statement.
seb is just being an attention-seeking trollette.
Yes it does. Please support your claim by actual science.
ROFLMAO.
So seb thinks that atmospheric CO2 concentration directly determines the ocean CO2 content..
Oh dearie me.. that is just arrant NONSENSE.
They can vary by +/- 50ppm at least !!
https://s19.postimg.cc/9t6x0ausz/sst-vs-co2.jpg
Seb says : “When the concentration of one gas in a mixture doubles, the volume increases accordingly.”
Seb, No warmist has yet tried to hoax us that adding CO2 to the atmosphere increases the volume of our atmosphere. This would be detectable as an increase in atmospheric pressure.
Since I notice that we above allow Wiki as an authority:
“The partial pressure of a gas is a measure of thermodynamic activity of the gas’s molecules. Gases dissolve, diffuse, and react according to their partial pressures, and not according to their concentrations in gas mixtures or liquids.” https://en.wikipedia.org/wiki/Partial_pressure
Penelope, I should probably have written “the share of the volume”.
Suppose you have a gas mixture with 50% gas #1 and 50% gas #2 and you are adding gas #2 to the mixture. Let’s say the mixture occupied 1 m^3 before and you add another 1 m^3 of gas #2. Then you have a gas mixture with a volume of 2 m^3 and gas #1 is 25% of that and gas #2 75%. Understood?
But we are talking about CO2 here which makes up only 0.04% of the atmosphere and the O2 part already was in the atmosphere before. Adding more of it hardly changes to total volume/mass of the atmosphere and thus you can safely say that increasing the concentration of CO2 is more or less the same as increasing the partial pressure of CO2 (percentage-wise).
YAWN
No indication of ANY pH change rom ALL measurements since 1900
https://s19.postimg.cc/ewnhcf8bn/ocean_PH_all_surface_readings.png
You continued IGNORANCE of the immense buffering against any possible pH change is bordering on the idiotic.. no, actually, well passed the idiotic..
You have NOTHING, seb
just empty mindless NIL-SCIENCE yapping.
AndyG55, you still don’t seem to get it. There is a supposed pH change of -0.08 that has been calculated from the amount of CO2 that is estimated to have been absorbed by the oceans between 1765 and 1994. This -0.08 figure is on top of every other change and variability.
The same is true for the effect CO2 has in the atmosphere. If CO2 would cause +1°C warming in a certain timespan, but it actually cooled 2°C then the temperatures are still 1°C warmer than they would be without the CO2 effect.
Is that so hard to understand? Instead you go on and rant something about pH not changing from 1900 on … talk something about buffering against pH change and yet post something about wildly different pH values around the globe at the same time. You are deeply conflicted and a pretty angry guy. I feel a bit sorry for you that you can’t help yourself to improve the hell out of this state …
“There is a supposed pH change “
ROFLMAO.
seb faceplants yet again !!
What is so hard for you to understand that you have ZERO-EVIDENCE of an CO2 causing warming, anywhere, anytime, and ZERO EVIDENECE of CO2 causing pH change ???
You really do live in a FICTICIOUS make-believe anti-science la-la-land seb.
“Instead you go on and rant something about pH not changing from 1900 on … talk something about buffering against pH change “
Yep, FACTS will always confuse you, seb.
Sorry that you choose to remain CLUELESS about chemistry and buffering,
Sorry that you choose to remain in fantasy land about changing pH, when ZERO trend is evident.
Sorry that you choose to remain wilfully IGNORANT, seb
.. but its not really a choice, is it seb.
.. its your ONLY option.
“The same is true for the effect CO2 has in the atmosphere. If CO2 would cause +1°C warming in a certain timespan, but it actually cooled 2°C then the temperatures are still 1°C warmer than they would be without the CO2 effect.”
And yet another meaningless load of seb anti-everything GIBBERISH !!
Fantasy piled high on AGW BS. !
“If CO2 would cause +1°C warming in a certain timespan”
again with the unproven suppository !
The FARCE is strong with this seb.
[…] https://notrickszone.com/2018/05/24/3-more-new-papers-expose-the-folly-of-ocean-acidification-claims/ […]
Near Hawaii, the surface pH is above 8.05, and in Alaska the surface pH is below 7.7 … and despite that, the marine environment in Alaska is much, much richer in life than the Hawaiian marine environment.
We often eat things like lemon juice, which has a pH of around two, which is neutral minus five pH units … whereas the most alkaline foods that we can tolerate have a pH of around eight, which is only one pH unit above neutral.
Fish often have a slimy kind of mucus that covers their entire bodies … to keep scales from slowly dissolving in the slightly alkaline ocean.
Some ecosystems such as coral reefs experience a daily range in pH that exceeds the modelled decrease in pH over the next century. And they thrive.
And some clueless clowns are worried about a mythical drop of 0.1 in pH value.
BIZZARE, and totally divorced from REALITY..
.. just like the whole of the AGW non-science.
While not being exactly the same, you can survive swimming in 15°C water for at least an hour. You can repeat this every day, no problem. But you will die, when you have to swim in 15° water for more than 24 hours.
Yeah, it’s an analogy. Totally irrelevant and stuff.
Read up what science says about ocean acidification and don’t expect a comment author, who points out that you are wrong, to explain it all to you. Do your homework yourself. Thank you.
And another MINDLESS IRRELEVENT analogy…
The first resort of a know-nothing seb.
Read up on what SCIENCE and CHEMISTRY say about pH, seb.
NOT what the AGW propagandists say.
I have done my homework..
.. but apparently, have NEVER even attended a science class.
Indeed.
But AngyG55,
< sarc-on >
modern coral have only become the predominant coral masses in our oceans in the last 20 million years or so, just how do you think such newly evolved creatures can possibly adapt to the changing climate? Surely they must just die out? And that will be all our fault, we didn’t look after them, I feel so guilty!
< sarc-off >
😉
😀
Ocean “acidification,” hahahahaha
“It is a myth that acidic waters necessarily kill aquatic life. Rain water is slightly acidic and many fresh water lagoons, swamps and reed beds are also acidic. Nevertheless, aquatic life flourishes in these wetlands.”
https://carbon-sense.com/2012/05/13/acid-ocean-bogeyman/
“Ocean Acidification is little more than a money-making scam for grant-troughing scientists who couldn’t find anything more productive to do with their semi-worthless environmental science degrees.”
http://www.breitbart.com/london/2017/01/09/delingpole-how-i-totally-crushed-the-ocean-acidification-alarmist-loons/
Here’s an objective review of “Ocean Acidification” research, what is wrong with it and why we need to be skeptical of hysterical activists.
“In this introduction, I present a brief overview of the history of research on OA, call for a heightened level of organized (academic) scepticism to be applied to the body of work on OA, and briefly present the 44 contributions that appear in this theme issue. ”
https://academic.oup.com/icesjms/article/73/3/529/2459146
I’m amazed that the existence of thousands of undersea volcanoes tends to be ignored. I’ve actually observed CO2 fumaroles. Surely volcanic activity would have a very significant effect on the temperature and the pH of the oceans.